Treatment of severe COVID-19 patients with either low- or high-volume of convalescent plasma versus standard of care: A multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI)
et al., The Lancet Regional Health - Americas, doi:10.1016/j.lana.2022.100216, COOP-COVID-19-MCTI, NCT04415086, Jun 2022
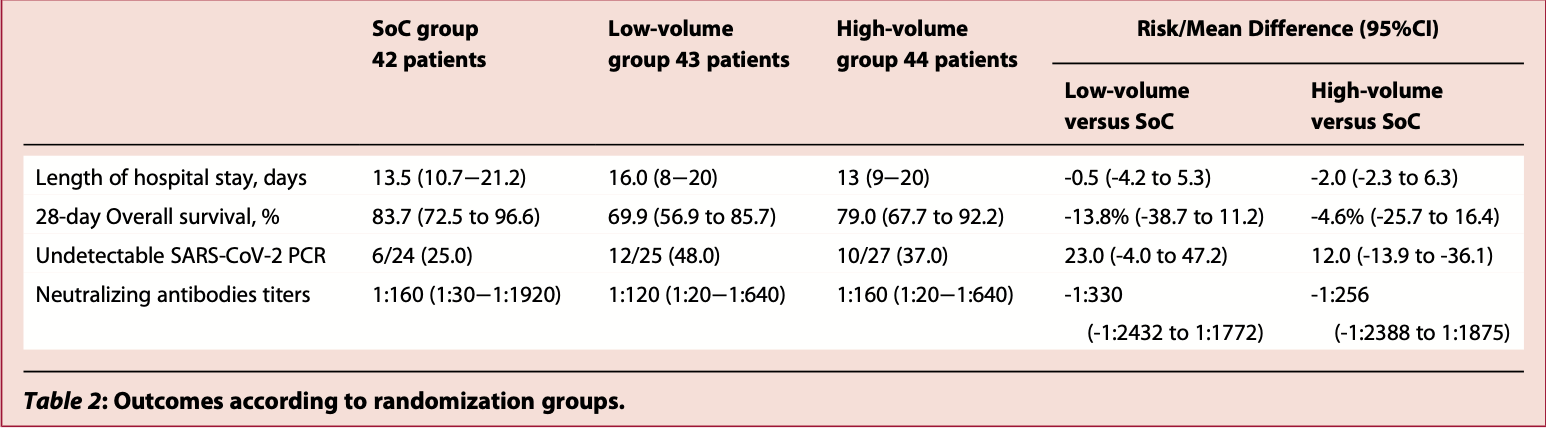
RCT 129 severe COVID-19 patients in Brazil, showing no significant difference in outcomes with convalescent plasma.
|
risk of death, 51.7% higher, RR 1.52, p = 0.37, treatment 22 of 87 (25.3%), control 7 of 42 (16.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Song et al., 30 Jun 2022, Randomized Controlled Trial, Brazil, peer-reviewed, median age 61.0, 20 authors, study period 2 June, 2020 - 18 November, 2020, average treatment delay 8.0 days, trial NCT04415086 (history) (COOP-COVID-19-MCTI).
Contact: esper.kallas@usp.br.
Treatment of severe COVID-19 patients with either low- or high-volume of convalescent plasma versus standard of care: A multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI)
The Lancet Regional Health - Americas, doi:10.1016/j.lana.2022.100216
Background Administration of convalescent plasma may serve as an adjunct to supportive treatment to prevent COVID-19 progression and death. We aimed to evaluate the efficacy and safety of 2 volumes of intravenous convalescent plasma (CP) with high antibody titers for the treatment of severe cases of COVID-19. Methods We conducted a Bayesian, randomized, open-label, multicenter, controlled clinical trial in 7 Brazilian hospitals. Adults admitted to hospital with positive RT-PCR for SARS-CoV2, within 10 days of the symptom onset, were eligible. Patients were randomly assigned (1:1:1) to receive standard of care (SoC) alone, or in combination with 200 mL (150−300 mL) of CP (Low-volume), or 400 mL (300−600 mL) of CP (High-volume); infusion had to be performed within 24 h of randomization. Randomization was centralized, stratified by center. The primary outcome was the time until clinical improvement up to day 28, measured by the WHO ten-point scale, assessed in the intention-to-treat population. Interim and terminal analyses were performed in a Bayesian framework. Trial registered at ClinicalTrials.gov: NCT04415086.
TV, LSM, JCF, CSL, and FCB led the implementation of the study. SC did the statistical plan and analysis and SC, ATWS, VIAS, NBC, and DGA have verified the underlying data. RRGM, ELD, CPS, RM, DBA, CSL, and EDC generated laboratory data. ATWS, VR, SC, VIAS, and EGK drafted the report. All other authors contributed to the implementation and data collection. All authors reviewed and approved the final report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary materials Supplementary material associated with this article can be found in the online version at doi:10.1016/j. lana.2022.100216.
References
Abani, Abbas, Abbas, Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, The Lancet
Avendaño-Sol A, Inez, Muñez-Rubio, A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest
B Egin, Callum, Jamula, Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med, doi:10.1038/s41591-021-01488-2
Bar, Shaw, Choi, A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia, J Clin Invest
Berry, Bayesian clinical trials, Nat Rev Drug Discov
Casadevall, Henderson, Joyner, SARS-CoV-2 variants and convalescent plasma: reality, fallacies, and opportunities, J Clin Invest
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med
Cheng, Wong, Soo, Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun
Hamilton, Lee, Arnold, Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial, Int J Infect Dis
Harrell, Lindsell, Statistical design and analysis plan for sequential parallel-group RCT for COVID-19
Horby, Mafham, Recovery, Group, RECOVERY): a randomised, controlled, openlabel, platform trial, doi:10.1101/2021.06.15.21258542
Hung, To, Lee, Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin Infect Dis
Hung, To, Lee, Hyperimmune IV immunoglobulin treatment, Chest
Joyner, Bruno, Klassen, Safety update, Mayo Clin Proc
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med
Korley, Durkalski-Mauldin, Yeatts, Early convalescent plasma for high-risk outpatients with Covid-19, N Engl J Med
Kunze, Johnson, Van Helmond, Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors, Nat Commun
K€ Orper, Weiss, Zickler, Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19, J Clin Invest
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and lifethreatening COVID-19: a randomized clinical trial, JAMA
Libster, Erez Marc, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis
Matthay, Thompson, Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties, Lancet Respir Med
Menichetti, Popoli, Puopolo, Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial, JAMA Netw Open
O'donnell, Grinsztejn, Cummings, A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J Clin Invest
Ryan, Bruce, Metcalfe, Using Bayesian adaptive designs to improve phase III trials: a respiratory care example, BMC Med Res Methodol, doi:10.1186/s12874-019-0739-3
Salazar, Christensen, Graviss, Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality, Am J Pathol
Santos, Proença, Leal, Marra, Yokoyama et al., References 1 WHO Coronavirus (COVID-19) dashboard
Sekine, Arns, Fabro, Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial, Eur Respir J
Simonovich, Pratx, Scibona, A Randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med
Tworek, Jaro N K, Uszy Nska-Ka»u_ Za, Convalescent plasma treatment is associated with lower mortality and better outcomes in high-risk COVID-19 patients − propensity-score matched case-control study, Int J Infect Dis
Van Griensven, Edwards, De Lamballerie, Evaluation of convalescent plasma for Ebola virus disease in Guinea, N Engl J Med
Von Cube, Schumacher, Wolkewitz, Causal inference with multistate models-estimands and estimators of the population attributable fraction, J R Stat Soc Ser A Stat Soc
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Zhou, Zhong, Guan, Treatment with convalescent plasma for influenza A (H5N1) infection, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.lana.2022.100216",
"ISSN": [
"2667-193X"
],
"URL": "http://dx.doi.org/10.1016/j.lana.2022.100216",
"alternative-id": [
"S2667193X22000333"
],
"article-number": "100216",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Treatment of severe COVID-19 patients with either low- or high-volume of convalescent plasma versus standard of care: A multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI)"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Regional Health - Americas"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.lana.2022.100216"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Song",
"given": "Alice T.W.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rocha",
"given": "Vanderson",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendrone-Júnior",
"given": "Alfredo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calado",
"given": "Rodrigo T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Santis",
"given": "Gil C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benites",
"given": "Bruno D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costa-Lima",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vargas",
"given": "Taiani",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3059-851X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marques",
"given": "Leonardo S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandes",
"given": "Juliana C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breda",
"given": "Felipe C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wendel",
"given": "Silvano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fachini",
"given": "Roberta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizzo",
"given": "Luiz V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kutner",
"given": "José Mauro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6660-3088",
"affiliation": [],
"authenticated-orcid": false,
"family": "Avelino-Silva",
"given": "Vivian I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Machado",
"given": "Rafael R.G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Durigon",
"given": "Edison L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chevret",
"given": "Sylvie",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2026-6925",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kallas",
"given": "Esper G.",
"sequence": "additional"
}
],
"container-title": "The Lancet Regional Health - Americas",
"container-title-short": "The Lancet Regional Health - Americas",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
3,
15
]
],
"date-time": "2022-03-15T18:56:10Z",
"timestamp": 1647370570000
},
"deposited": {
"date-parts": [
[
2022,
7,
24
]
],
"date-time": "2022-07-24T18:58:21Z",
"timestamp": 1658689101000
},
"indexed": {
"date-parts": [
[
2022,
7,
28
]
],
"date-time": "2022-07-28T09:49:48Z",
"timestamp": 1659001788269
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2022,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
1
]
],
"date-time": "2022-06-01T00:00:00Z",
"timestamp": 1654041600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
15
]
],
"date-time": "2022-02-15T00:00:00Z",
"timestamp": 1644883200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2667193X22000333?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2667193X22000333?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100216",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
6
]
]
},
"published-print": {
"date-parts": [
[
2022,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.lana.2022.100216_bib0001",
"unstructured": "WHO Coronavirus (COVID-19) dashboard. Updated August 18, 2021. Available at https://covid19.who.int."
},
{
"DOI": "10.1056/NEJMoa2031893",
"article-title": "Convalescent plasma antibody levels and the risk of death from Covid-19",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "1015",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0002",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0003",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0004",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"article-title": "Use of convalescent plasma therapy in SARS patients in Hong Kong",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "10.1016/j.lana.2022.100216_bib0005",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.1093/cid/ciq106",
"article-title": "Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.lana.2022.100216_bib0006",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.1378/chest.12-2907",
"article-title": "Hyperimmune IV immunoglobulin treatment",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "464",
"journal-title": "Chest",
"key": "10.1016/j.lana.2022.100216_bib0007",
"volume": "144",
"year": "2013"
},
{
"DOI": "10.1056/NEJMoa1511812",
"article-title": "Evaluation of convalescent plasma for Ebola virus disease in Guinea",
"author": "van Griensven",
"doi-asserted-by": "crossref",
"first-page": "33",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0008",
"volume": "374",
"year": "2016"
},
{
"DOI": "10.1056/NEJMc070359",
"article-title": "Treatment with convalescent plasma for influenza A (H5N1) infection",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1450",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0009",
"volume": "357",
"year": "2007"
},
{
"key": "10.1016/j.lana.2022.100216_bib0010",
"unstructured": "Food and Drug Administration (FDA). Recommendations for investigational COVID-19 convalescent plasma. Available at: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds. Accessed August 18, 2021."
},
{
"key": "10.1016/j.lana.2022.100216_bib0011",
"unstructured": "Ministério da Saúde, Agência Nacional de Vigilância Sanitária. Nota Técnica No 21/2020-CGSH/DAET/SAES/MS. Available at https://www.gov.br/anvisa/pt-br/arquivos-noticias-anvisa/666json-file-1. Accessed September 10, 2021."
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"author": "Marshall",
"doi-asserted-by": "crossref",
"first-page": "e192",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.lana.2022.100216_bib0012",
"volume": "20",
"year": "2020"
},
{
"key": "10.1016/j.lana.2022.100216_bib0013",
"unstructured": "Working Party for Haemovigilance. International society of blood transfusion/internation haemovigilance network. Available at https://www.isbtweb.org/fileadmin/user_upload/Proposed_definitions_2011_surveillance_non_infectious_adverse_reactions_haemovigilance_incl_TRALI_correction_2013_TACO_correction_2018.pdf with the updated TACO definition at https://www.isbtweb.org/fileadmin/user_upload/TACO_2018_definition_March_2019.pdf. Accessed April 20, 2020."
},
{
"DOI": "10.1186/s12874-019-0739-3",
"article-title": "Using Bayesian adaptive designs to improve phase III trials: a respiratory care example",
"author": "Ryan",
"doi-asserted-by": "crossref",
"journal-title": "BMC Med Res Methodol",
"key": "10.1016/j.lana.2022.100216_bib0014",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1038/nrd1927",
"article-title": "Bayesian clinical trials",
"author": "Berry",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "Nat Rev Drug Discov",
"key": "10.1016/j.lana.2022.100216_bib0015",
"volume": "5",
"year": "2006"
},
{
"key": "10.1016/j.lana.2022.100216_bib0016",
"unstructured": "Harrell F., Lindsell C. Statistical design and analysis plan for sequential parallel-group RCT for COVID-19. Available at: http://hbiostat.org/proj/covid19/bayesplan.html. Accessed March 30, 2020."
},
{
"DOI": "10.1111/rssa.12486",
"article-title": "Causal inference with multistate models—estimands and estimators of the population attributable fraction",
"author": "von Cube",
"doi-asserted-by": "crossref",
"first-page": "1479",
"journal-title": "J R Stat Soc Ser A Stat Soc",
"key": "10.1016/j.lana.2022.100216_bib0017",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"article-title": "Safety update",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "1888",
"journal-title": "Mayo Clin Proc",
"key": "10.1016/j.lana.2022.100216_bib0018",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/j.ajpath.2020.08.001",
"article-title": "Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality",
"author": "Salazar",
"doi-asserted-by": "crossref",
"first-page": "2290",
"journal-title": "Am J Pathol",
"key": "10.1016/j.lana.2022.100216_bib0019",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2021.02.054",
"article-title": "Convalescent plasma treatment is associated with lower mortality and better outcomes in high-risk COVID-19 patients – propensity-score matched case-control study",
"author": "Tworek",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.lana.2022.100216_bib0020",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "460",
"journal-title": "JAMA",
"key": "10.1016/j.lana.2022.100216_bib0021",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"article-title": "Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"first-page": "3189",
"journal-title": "Nat Commun",
"key": "10.1016/j.lana.2022.100216_bib0022",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A Randomized trial of convalescent plasma in Covid-19 severe pneumonia",
"author": "Simonovich",
"doi-asserted-by": "crossref",
"first-page": "619",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0023",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.36246",
"article-title": "Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial",
"author": "Menichetti",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.lana.2022.100216_bib0024",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial",
"author": "Abani",
"doi-asserted-by": "crossref",
"first-page": "2049",
"journal-title": "The Lancet",
"key": "10.1016/j.lana.2022.100216_bib0025",
"volume": "397",
"year": "2021"
},
{
"article-title": "Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial",
"author": "Sekine",
"journal-title": "Eur Respir J",
"key": "10.1016/j.lana.2022.100216_bib0026",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"article-title": "Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial",
"author": "Bégin",
"doi-asserted-by": "crossref",
"journal-title": "Nat Med",
"key": "10.1016/j.lana.2022.100216_bib0027",
"year": "2021"
},
{
"DOI": "10.1172/JCI152264",
"article-title": "Results of the CAPSID randomized trial for high-dose convalescent plasma in patients with severe COVID-19",
"author": "Körper",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.lana.2022.100216_bib0028",
"volume": "131",
"year": "2021"
},
{
"article-title": "A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19",
"author": "O'Donnell",
"journal-title": "J Clin Invest",
"key": "10.1016/j.lana.2022.100216_bib0029",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early convalescent plasma for high-risk outpatients with Covid-19",
"author": "Korley",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0030",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"article-title": "Early high-titer plasma therapy to prevent severe Covid-19 in older adults",
"author": "Libster",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "10.1016/j.lana.2022.100216_bib0031",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.06.034",
"article-title": "Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial",
"author": "Hamilton",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.lana.2022.100216_bib0032",
"volume": "109",
"year": "2021"
},
{
"DOI": "10.1172/JCI152740",
"article-title": "A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia",
"author": "Avendaño-Solá",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.lana.2022.100216_bib0033",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1172/JCI155114",
"article-title": "A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia",
"author": "Bar",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.lana.2022.100216_bib0034",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-25113-5",
"article-title": "Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors",
"author": "Kunze",
"doi-asserted-by": "crossref",
"first-page": "4864",
"journal-title": "Nat Commun",
"key": "10.1016/j.lana.2022.100216_bib0035",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1101/2021.06.15.21258542",
"doi-asserted-by": "crossref",
"key": "10.1016/j.lana.2022.100216_bib0036",
"unstructured": "Horby PW, Mafham M, RECOVERY Collaborative Group, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Preprint, Infectious Diseases (except HIV/AIDS). Epub ahead of print, 16 June 2021. DOI:10.1101/2021.06.15.21258542"
},
{
"DOI": "10.1172/JCI148832",
"article-title": "SARS-CoV-2 variants and convalescent plasma: reality, fallacies, and opportunities",
"author": "Casadevall",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "10.1016/j.lana.2022.100216_bib0037",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30503-8",
"article-title": "Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties",
"author": "Matthay",
"doi-asserted-by": "crossref",
"first-page": "1170",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.lana.2022.100216_bib0038",
"volume": "8",
"year": "2020"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2667193X22000333"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Treatment of severe COVID-19 patients with either low- or high-volume of convalescent plasma versus standard of care: A multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "10"
}