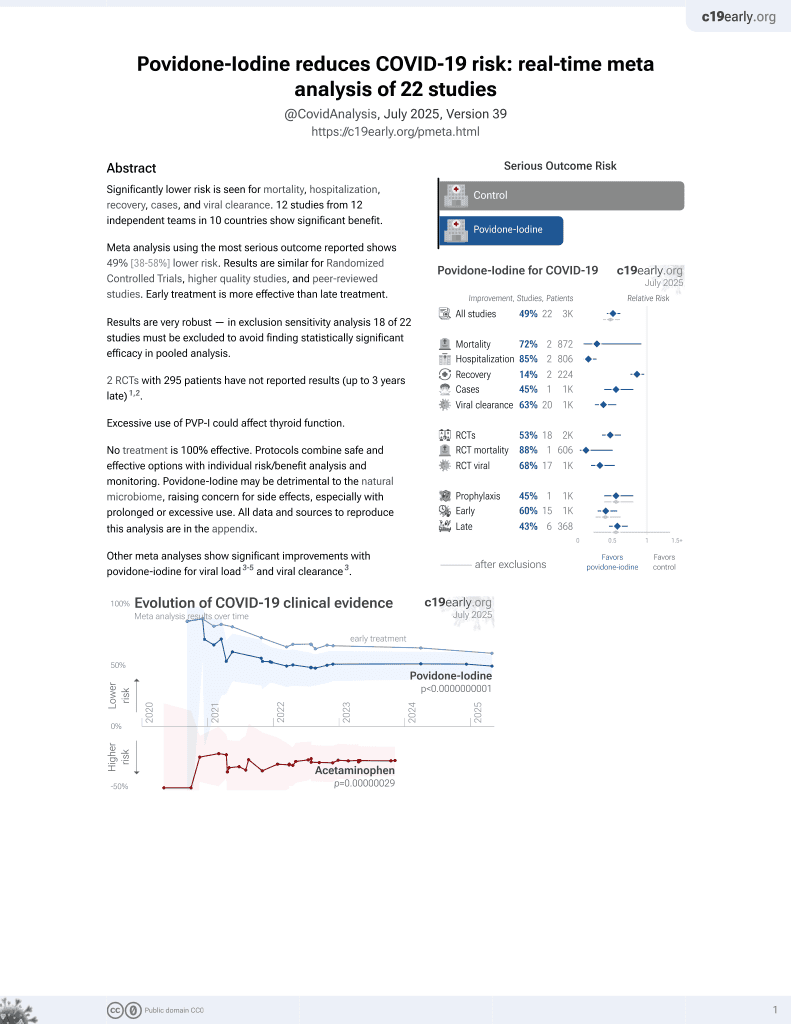
A pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx
et al., medRxiv, doi:10.1101/2022.08.18.22278340, TCTR20210125002, Aug 2022
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small single-arm trial testing short-term viral load change after a single administration of three puffs of 0.4% PVP-I, showing lower viral titer at 3 minutes and 4 hours, not reaching statistical significance. Authors note that one reason for the lower change compared to in vitro results is that the spray administration may be less effective.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
This study is excluded in the after exclusion results of meta-analysis:
study only provides short-term viral load results.
|
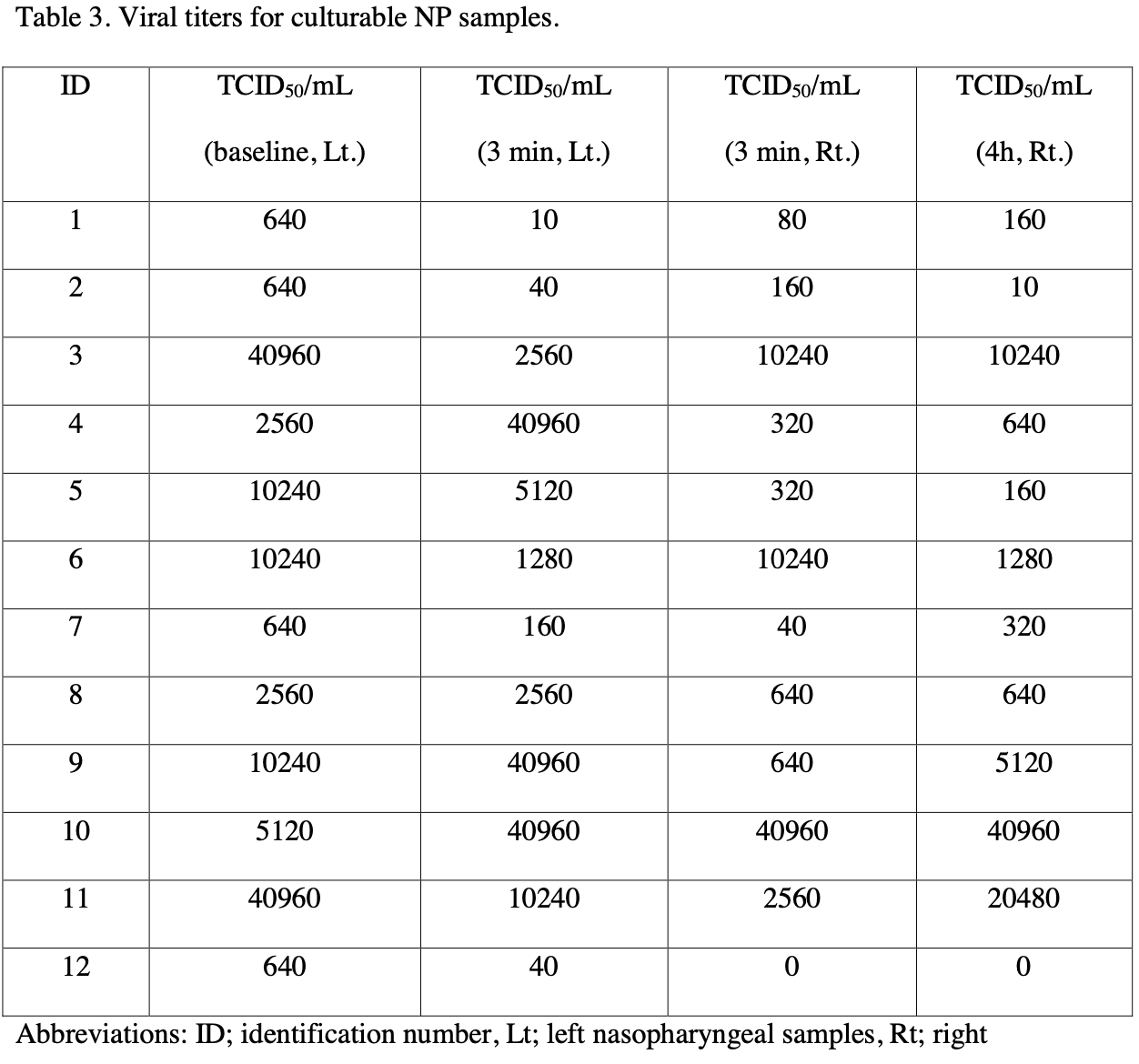
viral load, 33.3% lower, relative load 0.67, p = 0.58, after median 2560 IQR 17790 n=12, before median 3840 IQR 9600 n=12, before values
640.0 640.0 40960.0 2560.0 10240.0 10240.0 640.0 2560.0 10240.0 5120.0 40960.0 640.0, after values
10.0 40.0 2560.0 40960.0 5120.0 1280.0 160.0 2560.0 40960.0 40960.0 10240.0 40.0, relative median viral titer, 3 min, left vs. baseline, Mann-Whitney, Table 3.
|
|
viral load, 87.5% lower, relative load 0.12, p = 0.04, after median 480 IQR 4340 n=12, before median 3840 IQR 9600 n=12, before values
640.0 640.0 40960.0 2560.0 10240.0 10240.0 640.0 2560.0 10240.0 5120.0 40960.0 640.0, after values
80.0 160.0 10240.0 320.0 320.0 10240.0 40.0 640.0 640.0 40960.0 2560.0 0.0, relative median viral titer, 3 min, right vs. baseline, Mann-Whitney, Table 3.
|
|
viral load, 83.3% lower, relative load 0.17, p = 0.11, after median 640 IQR 6240 n=12, before median 3840 IQR 9600 n=12, before values
640.0 640.0 40960.0 2560.0 10240.0 10240.0 640.0 2560.0 10240.0 5120.0 40960.0 640.0, after values
160.0 10.0 10240.0 640.0 160.0 1280.0 320.0 640.0 5120.0 40960.0 20480.0 0.0, relative median viral titer, 4 hours, right vs. baseline, Mann-Whitney, Table 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sirijatuphat et al., 22 Aug 2022, prospective, Thailand, preprint, median age 34.0, 4 authors, study period 15 February, 2021 - 15 March, 2021, trial TCTR20210125002.
Contact: amorn.lee@mahidol.ac.th.
A pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx
doi:10.1101/2022.08.18.22278340
We studied the virucidal efficacy of 0.4% povidone-iodine (PVP-I) nasal spray against SARS-CoV-2 in the patients' nasopharynx at 3 minutes and 4 hours after PVP-I exposure. We used an open-label, before and after design, single arm pilot study of adult patients with RT-PCRconfirmed COVID-19 within 24 hours. All patients received three puffs of 0.4% PVP-I nasal spray in each nostril. Nasopharyngeal (NP) swabs were collected before the PVP-I spray (baseline, left NP samples), and at 3 minutes (left and right NP samples) and 4 hours post-PVP-I spray (right NP samples). All swabs were coded to blind assessors and transported to diagnostic laboratory and tested by RT-PCR and cultured to measure the viable SARS-CoV-2 within 24 hours after collection. Fourteen patients were enrolled but viable SARS-CoV-2 was cultured from 12 patients (85.7%). The median viral titer at baseline was 3.5 log TCID 50 /mL (IQR 2.8-4.0 log TCID 50 /mL). At 3 minutes post-PVP-I spray via the left nostril, viral titers were reduced in 8 patients (66.7%). At 3 minutes post-PVP-I, the median viral titer was 3.4 log TCID 50 /mL (IQR 1.8-4.4 log TCID 50 /mL) (P=0.162). At 4 hours post-PVP-I spray via the right nostril, 6 of 11 patients (54.5%) had either the same or minimal change in viral titers. The median viral titer 3 minutes post-PVP-I spray was 2.7 log TCID 50 /mL (IQR 2.0-3.9 log TCID 50 /mL). Four hours post-PVP-I spray the median titer was 2.8 log TCID 50 /mL (IQR 2.2-3.9 log TCID 50 /mL) (P=0.704). No adverse effects of 0.4% PVP-I nasal spray were detected. We concluded that 0.4% PVP-I nasal spray demonstrated minimal virucidal efficacy at 3 minutes post-exposure. At 4 hours post-exposure, the viral titer was considerably unchanged from baseline in 10 cases. The 0.4% PVP-I nasal spray showed poor virucidal activity and is unlikely to reduce transmission of SARS-CoV-2 in prophylaxis use. .
Discussion The TCID 50 /mL of the 12 patients with culturable NP samples varied from 640 to 40,960, which was not related to either disease severity or the onset of clinical symptoms when NP swabs were taken. Because all cases recovered uneventfully, the initial values of TCID 50 /ml did not predict the treatment outcome. However, the sample size was small and we did not plan to study the relationship between the initial viral titers and disease severity or clinical outcome. We found a median one-fold reduction of SARS-CoV-2 viral titer from nasopharyngeal swabs at 3 minute post-PVP-I exposure in the left side of nasopharynx. This was in marked contrast to in vitro studies that reported as much as a 100-fold reduction in viral titer [5] . At 4 hour post-exposure to 0.4% PVP-I nasal spray, the viral titer in the right side of nasopharynx was unchanged from baseline in 10 cases. Although the in vitro rapid activity of PVP-I against SARS-CoV-2 has been established in several studies [5] [6] [7] , evidence of in vivo activity in humans is limited. Two studies have reported that PVP-I administration into the upper aerodigestive tract of COVID-19 patients was associated with lower SARS-CoV-2 viral load and Ct values [12, 13] . However, other studies have not confirmed their results [14] [15] [16] . To measure potential reductions in viral shedding, we believe that RT-PCR alone is insufficient and viral culture is needed. Using viral culture, PVP-I administration probably..
References
Anderson, Sivalingam, Kang, Ananthanarayanan, Arumugam et al., Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease, Infect Dis Ther
Badu, Oyebola, Zahouli, Fagbamigbe, De Souza, SARS-CoV-2 Viral Shedding and Transmission Dynamics: Implications of WHO COVID-19 Discharge Guidelines, Front Med
Bidra, Pelletier, Westover, Frank, Brown et al., Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse, J Prosthodont
Casey-Bryars, Griffin, Mcaloon, Byrne, Madden et al., Presymptomatic transmission of SARS-CoV-2 infection: a secondary analysis using published data, BMJ Open
Covid-, Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21, Lancet
Dhand, Khatkar, Statulator: An online statistical calculator. Sample Size Calculator for Comparing Two Paired Proportions
Elzein, Sater, Fakhreddine, Hanna, Feghali et al., In vivo evaluation of the virucidal efficacy of Chlorhexidine and Povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial, J Evid Based Dent Pract
Ferrer, Barrueco, Martinez-Beneyto, Moreno, Ausina-Márquez et al., Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Sci Rep
Friedland, Tucker, Goodall, Julander, Meddenhall et al., In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray, Aust J Otolaryngol
Ghaddara, Kumar, Cadnum, Ng-Wong, Donskey, Efficacy of a povidone iodine preparation in reducing nasal methicillin-resistant Staphylococcus aureus in colonized patients, Am J Infect Control
Guenezan, Garcia, Strasters, Jousselin, Lévêque et al., Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: A randomized clinical trial, JAMA Otolaryngol Head Neck Surg
Kunno, Supawattanabodee, Sumanasrethakul, Wiriyasivaj, Kuratong et al., Comparison of Different Waves during the COVID-19 Pandemic: Retrospective Descriptive Study in Thailand, Adv Prev Med
Nagatake, Ahmed, Oishi, Prevention of respiratory infections by povidone-iodine gargle, Dermatology
Pelletier, Miller, Liang, Capriotti, In vitro efficacy of a povidone-iodine 0.4% and dexamethasone 0.1% suspension against ocular pathogens, J Cataract Refract Surg
Peng, Wang, Zhai, Weng, Feng, Effectiveness of preoperative decolonization with nasal povidone iodine in Chinese patients undergoing elective orthopedic surgery: a prospective cross-sectional study, Braz J Med Biol Res
Sasaki, Uemura, Sato, Toba, Sanaki et al., SARS-CoV-2 variants with mutations at the S1/S2 cleavage site are generated in vitro during propagation in TMPRSS2-deficient cells, PLoS Pathog
Seikai, Takada, Hasebe, Kajihara, Okuya et al., Gargling with povidone iodine has a short-term inhibitory effect on SARS-CoV-2 in patients with COVID-19, J Hosp Infect
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore, Infection
Shet, Hong, Igo, Cataldo, Bhaskar, In vitro evaluation of the virucidal activity of different povidone-iodine formulations against murine and human coronaviruses, Infect Dis Ther
Wang, Tan, Summary of the Detection Kits for SARS-CoV-2
Wojciechowska, Krajewski, Zub, Zatoński, Review of practical recommendations for otolaryngologists and head and neck surgeons during the COVID-19 pandemic, Auris Nasus Larynx
Zarabanda, Vukkadala, Phillips, Qian, Mfuh et al., The Effect of Povidone-Iodine Nasal Spray on Nasopharyngeal SARS-CoV-2 Viral Load: A Randomized Control Trial, Laryngoscope
DOI record:
{
"DOI": "10.1101/2022.08.18.22278340",
"URL": "http://dx.doi.org/10.1101/2022.08.18.22278340",
"abstract": "<jats:title>Abstract</jats:title><jats:p>We studied the virucidal efficacy of 0.4% povidone-iodine (PVP-I) nasal spray against SARS-CoV-2 in the patients’ nasopharynx at 3 minutes and 4 hours after PVP-I exposure. We used an open-label, before and after design, single arm pilot study of adult patients with RT-PCR-confirmed COVID-19 within 24 hours. All patients received three puffs of 0.4% PVP-I nasal spray in each nostril. Nasopharyngeal (NP) swabs were collected before the PVP-I spray (baseline, left NP samples), and at 3 minutes (left and right NP samples) and 4 hours post-PVP-I spray (right NP samples). All swabs were coded to blind assessors and transported to diagnostic laboratory and tested by RT-PCR and cultured to measure the viable SARS-CoV-2 within 24 hours after collection. Fourteen patients were enrolled but viable SARS-CoV-2 was cultured from 12 patients (85.7%). The median viral titer at baseline was 3.5 log TCID<jats:sub>50</jats:sub>/mL (IQR 2.8-4.0 log TCID<jats:sub>50</jats:sub>/mL). At 3 minutes post-PVP-I spray via the left nostril, viral titers were reduced in 8 patients (66.7%). At 3 minutes post-PVP-I, the median viral titer was 3.4 log TCID<jats:sub>50</jats:sub>/mL (IQR 1.8-4.4 log TCID<jats:sub>50</jats:sub>/mL) (<jats:italic>P</jats:italic>=0.162). At 4 hours post-PVP-I spray via the right nostril, 6 of 11 patients (54.5%) had either the same or minimal change in viral titers. The median viral titer 3 minutes post-PVP-I spray was 2.7 log TCID<jats:sub>50</jats:sub>/mL (IQR 2.0-3.9 log TCID<jats:sub>50</jats:sub>/mL). Four hours post-PVP-I spray the median titer was 2.8 log TCID<jats:sub>50</jats:sub>/mL (IQR 2.2-3.9 log TCID<jats:sub>50</jats:sub>/mL) (<jats:italic>P</jats:italic>=0.704). No adverse effects of 0.4% PVP-I nasal spray were detected. We concluded that 0.4% PVP-I nasal spray demonstrated minimal virucidal efficacy at 3 minutes post-exposure. At 4 hours post-exposure, the viral titer was considerably unchanged from baseline in 10 cases. The 0.4% PVP-I nasal spray showed poor virucidal activity and is unlikely to reduce transmission of SARS-CoV-2 in prophylaxis use.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
8,
22
]
]
},
"author": [
{
"affiliation": [],
"family": "Sirijatuphat",
"given": "Rujipas",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9865-4330",
"affiliation": [],
"authenticated-orcid": false,
"family": "Leelarasamee",
"given": "Amorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puangpet",
"given": "Thanapat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thitithanyanont",
"given": "Arunee",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
8,
23
]
],
"date-time": "2022-08-23T02:40:15Z",
"timestamp": 1661222415000
},
"deposited": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T18:26:50Z",
"timestamp": 1661365610000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T18:44:05Z",
"timestamp": 1661366645882
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8,
22
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.08.18.22278340",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
8,
22
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
8,
22
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2022082411251770000_2022.08.18.22278340v1.1",
"unstructured": "COVID-19 Excess Mortality Collaborators. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022:S0140-6736(21)02796-3."
},
{
"article-title": "SARS-CoV-2 Viral Shedding and Transmission Dynamics: Implications of WHO COVID-19 Discharge Guidelines",
"first-page": "648660",
"journal-title": "Front Med (Lausanne)",
"key": "2022082411251770000_2022.08.18.22278340v1.2",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-041240",
"doi-asserted-by": "publisher",
"key": "2022082411251770000_2022.08.18.22278340v1.3"
},
{
"DOI": "10.1016/j.jcrs.2010.11.028",
"doi-asserted-by": "publisher",
"key": "2022082411251770000_2022.08.18.22278340v1.4"
},
{
"DOI": "10.1007/s40121-020-00316-3",
"article-title": "Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease",
"doi-asserted-by": "crossref",
"first-page": "669",
"journal-title": "Infect Dis Ther",
"key": "2022082411251770000_2022.08.18.22278340v1.5",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1111/jopr.13209",
"article-title": "Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "J Prosthodont",
"key": "2022082411251770000_2022.08.18.22278340v1.6",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1007/s40121-021-00536-1",
"article-title": "In vitro evaluation of the virucidal activity of different povidone-iodine formulations against murine and human coronaviruses",
"doi-asserted-by": "crossref",
"first-page": "2777",
"journal-title": "Infect Dis Ther",
"key": "2022082411251770000_2022.08.18.22278340v1.7",
"volume": "10",
"year": "2021"
},
{
"article-title": "Prevention of respiratory infections by povidone-iodine gargle",
"first-page": "32",
"issue": "Suppl 1",
"journal-title": "Dermatology",
"key": "2022082411251770000_2022.08.18.22278340v1.8",
"volume": "204",
"year": "2002"
},
{
"DOI": "10.1016/j.ajic.2019.09.014",
"doi-asserted-by": "publisher",
"key": "2022082411251770000_2022.08.18.22278340v1.9"
},
{
"DOI": "10.1590/1414-431X20176736",
"doi-asserted-by": "publisher",
"key": "2022082411251770000_2022.08.18.22278340v1.10"
},
{
"DOI": "10.1016/j.anl.2020.05.022",
"article-title": "Review of practical recommendations for otolaryngologists and head and neck surgeons during the COVID-19 pandemic",
"doi-asserted-by": "crossref",
"first-page": "544",
"journal-title": "Auris Nasus Larynx",
"key": "2022082411251770000_2022.08.18.22278340v1.11",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1007/s15010-020-01563-9",
"article-title": "Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "Infection",
"key": "2022082411251770000_2022.08.18.22278340v1.12",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1016/j.jebdp.2021.101584",
"article-title": "In vivo evaluation of the virucidal efficacy of Chlorhexidine and Povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial",
"doi-asserted-by": "crossref",
"first-page": "101584",
"journal-title": "J Evid Based Dent Pract",
"key": "2022082411251770000_2022.08.18.22278340v1.13",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1001/jamaoto.2020.5490",
"article-title": "Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: A randomized clinical trial",
"doi-asserted-by": "crossref",
"first-page": "400",
"journal-title": "JAMA Otolaryngol Head Neck Surg",
"key": "2022082411251770000_2022.08.18.22278340v1.14",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1002/lary.29935",
"doi-asserted-by": "publisher",
"key": "2022082411251770000_2022.08.18.22278340v1.15"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"article-title": "Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "24392",
"journal-title": "Sci Rep",
"key": "2022082411251770000_2022.08.18.22278340v1.16",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1007/s12250-020-00331-1",
"article-title": "Summary of the Detection Kits for SARS-CoV-2 Approved by the National Medical Products Administration of China and Their Application for Diagnosis of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "699",
"journal-title": "Virol Sin",
"key": "2022082411251770000_2022.08.18.22278340v1.17",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1009233",
"doi-asserted-by": "publisher",
"key": "2022082411251770000_2022.08.18.22278340v1.18"
},
{
"key": "2022082411251770000_2022.08.18.22278340v1.19",
"unstructured": "Dhand NK , Khatkar MS . (2014). Statulator: An online statistical calculator. Sample Size Calculator for Comparing Two Paired Proportions. Accessed 25 July 2022 at http://statulator.com/SampleSize/ss2PP.html"
},
{
"DOI": "10.21037/ajo-21-40",
"article-title": "In vivo (human) and in vitro inactivation of SARS-CoV-2 with 0.5% povidone-iodine nasal spray",
"doi-asserted-by": "crossref",
"first-page": "2",
"journal-title": "Aust J Otolaryngol",
"key": "2022082411251770000_2022.08.18.22278340v1.20",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.jhin.2022.01.001",
"doi-asserted-by": "crossref",
"key": "2022082411251770000_2022.08.18.22278340v1.21",
"unstructured": "Seikai T , Takada A , Hasebe A , Kajihara M , Okuya K , Sekiguchi Yamada T , et al. Gargling with povidone iodine has a short-term inhibitory effect on SARS-CoV-2 in patients with COVID-19. J Hosp Infect 2022: S0195-6701(22)00006-8."
},
{
"article-title": "Comparison of Different Waves during the COVID-19 Pandemic: Retrospective Descriptive Study in Thailand",
"first-page": "5807056",
"journal-title": "Adv Prev Med",
"key": "2022082411251770000_2022.08.18.22278340v1.22",
"volume": "2021",
"year": "2021"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.08.18.22278340"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "A pilot study of 0.4% povidone-iodine nasal spray to eradicate SARS-CoV-2 in the nasopharynx",
"type": "posted-content"
}