Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial
et al., Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2021.111526, BeeCovid, NCT04480593, Jun 2021
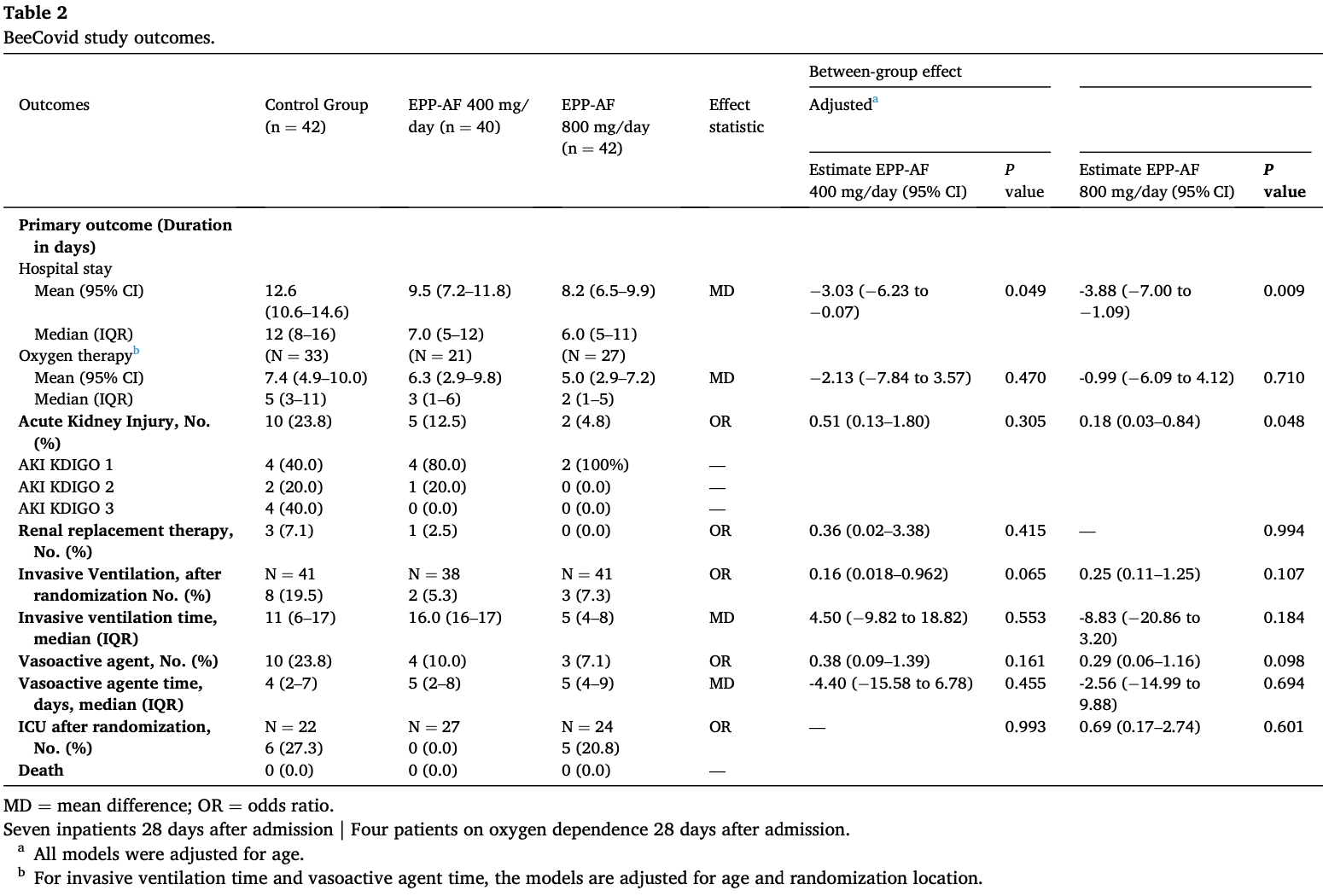
RCT 124 hospitalized COVID-19 patients in Brazil. The treatment groups received standardized green propolis extract (EPP-AF) at doses of 400mg/day or 800mg/day for 7 days, in addition to standard care. The EPP-AF groups had significantly shorter hospital stays post-intervention. The high dose EPP-AF group also had lower rates of acute kidney injury. No significant differences were seen for other outcomes like oxygen therapy duration or need for mechanical ventilation. The propolis adjunct treatment appeared safe with no discontinuations due to side effects.
|
risk of mechanical ventilation, 62.5% lower, RR 0.37, p = 0.19, treatment 3 of 41 (7.3%), control 8 of 41 (19.5%), NNT 8.2, 800mg/day.
|
|
risk of mechanical ventilation, 73.0% lower, RR 0.27, p = 0.09, treatment 2 of 38 (5.3%), control 8 of 41 (19.5%), NNT 7.0, 400mg/day.
|
|
risk of ICU admission, 23.6% lower, RR 0.76, p = 0.73, treatment 5 of 24 (20.8%), control 6 of 22 (27.3%), NNT 16, 800mg/day.
|
|
risk of ICU admission, 93.0% lower, RR 0.07, p = 0.005, treatment 0 of 27 (0.0%), control 6 of 22 (27.3%), NNT 3.7, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), 400mg/day.
|
|
hospitalization time, 34.9% lower, relative time 0.65, p = 0.001, treatment mean 8.2 (±5.62) n=42, control mean 12.6 (±6.61) n=42, 800mg/day.
|
|
hospitalization time, 24.6% lower, relative time 0.75, p = 0.049, treatment mean 9.5 (±7.42) n=40, control mean 12.6 (±6.61) n=42, 400mg/day.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Silveira et al., 30 Jun 2021, Randomized Controlled Trial, Brazil, peer-reviewed, 25 authors, trial NCT04480593 (history) (BeeCovid).
Contact: marceloadsilveira@gmail.com.
Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial
Biomedicine & Pharmacotherapy, doi:10.1016/j.biopha.2021.111526
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) promotes challenging immune and inflammatory phenomena. Though various therapeutic possibilities have been tested against coronavirus disease 2019 (COVID-19), the most adequate treatment has not yet been established. Propolis is a natural product with considerable evidence of immunoregulatory and anti-inflammatory activities, and experimental data point to potential against viral targets. We hypothesized that propolis can reduce the negative effects of COVID-19. Methods: In a randomized, controlled, open-label, single-center trial, hospitalized adult COVID-19 patients were treated with a standardized green propolis extract (EPP-AF® ) as an adjunct therapy. Patients were allocated to receive standard care plus an oral dose of 400 mg or 800 mg/day of green propolis for seven days, or standard care alone. Standard care included all necessary interventions, as determined by the attending physician. The primary end point was the time to clinical improvement, defined as the length of hospital stay or oxygen therapy dependency duration. Secondary outcomes included acute kidney injury and need for intensive care or vasoactive drugs. Patients were followed for 28 days after admission. Results: We enrolled 124 patients; 40 were assigned to EPP-AF® 400 mg/day, 42 to EPP-AF® 800 mg/day, and 42 to the control group. The length of hospital stay post-intervention was shorter in both propolis groups than in the control group; lower dose, median 7 days versus 12 days (95% confidence interval [CI] -6.23 to -0.07; p = 0.049) and higher dose, median 6 days versus 12 days (95% CI -7.00 to -1.09; p = 0.009). Propolis did not significantly affect the need for oxygen supplementation. In the high dose propolis group, there was a lower rate
Author Contributions MADS designed the trial and was the principal investigator, with overall responsibility for conducting the trial and for medical oversight of trial implementation and wrote the final report. RHP, SNFG, AVAM, JGR, and PBPB were responsible for the study design with the principal investigator. DDJ, AAB and RHP wrote the final report. JCR and TCS were responsible for analyzing the data. EBSG, RLASM, and TCA were responsible for coordinating and organizing the participants' data. SPS, LFMRC, MMDG, MBT, MHCAS, MOS, ML, SSU, and FSA contributed to the clinical and operational implementation of the trial. All authors contributed to trial design and interpretation of data and reviewed the final report.
Conflict of interest statement Dr. Berretta is an employee of Apis Flora. Dr. Silveira and all the other authors, except for Dr. Berretta and Dr. De Jong, are employed by the São Rafael hospital, which is part of the D'Or Institute for Research and Education.
Appendix A. Supporting information Supplementary data associated with this article can be found in the online version at doi:10.1016/j.biopha.2021.111526.
References
-F. Chan, Cheung, Sze, The immunomodulatory and anticancer properties of propolis, Clin. Rev. Allerg. Immunol, doi:10.1007/s12016-012-8322-2
Al Naggar, Giesy, Abdel-Daim, Javed Ansari, Al-Kahtani et al., Fighting against the second wave of COVID-19: can honeybee products help protect against the pandemic? Saudi, J. Biol. Sci, doi:10.1016/j.sjbs.2020.12.031
Ali, Daoud, Mohamed, Salim, Yessayan et al., Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis, Ren. Fail, doi:10.1080/0886022X.2020.1756323
Bachevski, Damevska, Simeonovski, Dimova, Back to the basics: propolis and COVID-19, Dermatol. Ther, doi:10.1111/dth.13780
Bankova, Chemical diversity of propolis and the problem of standardization, J. Ethnopharmacol, doi:10.1016/j.jep.2005.05.004
Batlle, Soler, Sparks, Hiremath, South et al., COVID-19 and ACE2 in Cardiovascular, lung, and kidney working group. acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology, J. Am. Soc. Nephrol, doi:10.1681/ASN.2020040419
Berretta, Arruda, Miguel, Baptista, Nascimento et al., Functional properties of brazilian propolis: from chemical composition until the market, doi:10.5772/65932
Berretta, Nascimento, Bueno, Vaz, Marchetti, Propolis standardized extract (EPP-AF(R)), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds, Int. J. Biol. Sci, doi:10.7150/ijbs.3641
Berretta, Silveira, Cóndor Capcha, Jong, Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease, Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110622
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, New Engl. J. Med, doi:10.1056/NEJMoa2001282
Cusinato, Martinez, Cintra, Filgueira, Berretta et al., Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF®) using an in vivo cocktail approach, J. Ethnopharmacol, doi:10.1016/j.jep.2019.112174
Cutler, Summers, The COVID-19 pandemic and the $16 Trillion Virus, JAMA, doi:10.1001/jama.2020.19759
Da, Xu, Wang, Li, Lu et al., Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer, Anal. Cell. Pathol, doi:10.1155/2019/1907698
De Miranda, Nascimento Lanna, De Paula, De Souza, Felipetto et al., Hydroalcoholic extract of Brazilian green propolis modulates inflammatory process in mice submitted to a low protein diet, Biomed. Pharmacother, doi:10.1016/j.biopha.2018.10.116
Diniz, Lorencini, Berretta, Cintra, Lia et al., Antioxidant effect of standardized extract of propolis (EPP-AF®) in healthy volunteers: a "before and after" clinical study, Evid. Based Complement. Altern. Med, doi:10.1155/2020/7538232
Elwakil, Shaaban, Bekhit, El-Naggar, Olama, Potential anti-COVID-19 activity of Egyptian propolis using computational modeling, Future Virol, doi:10.2217/fvl-2020-0329
Esposito, Garzarella, Bocchino, D'avino, Caruso et al., A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): a monocentric, randomized, double-blind, placebo-controlled clinical trial, Phytomedicine, doi:10.1016/j.phymed.2020.153368
Fiorini, Scorza, De Almeida, Fonseca, Finsterer et al., Antiviral activity of Brazilian green propolis extract against SARS-CoV-2 (severe acute respiratory syndrome -coronavirus 2) infection: case report and review, Clinics, doi:10.6061/clinics/2021/e2357
Forrest, Rayner, Park, Mills, Early treatment of COVID-19 disease: a missed opportunity, Infect. Dis. Ther, doi:10.1007/s40121-020-00349-8
Frozza, Santos, Rufatto, Minetto, Scariot et al., Antitumor activity of Brazilian red propolis fractions against Hep-2 cancer cell line, Biomed. Pharmacother, doi:10.1016/j.biopha.2017.05.027
Galeotti, Maccari, Fachini, Volpi, Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products, Foods, doi:10.3390/foods7030041
Gordon, Jang, Bouhaddou, Xu, Obernier et al., SARS-CoV-2, protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, New Engl. J. Med, doi:10.1056/NEJMoa2002032
Gupta, Coca, Chan, Melamed, Brenner et al., the STOP-COVID Investigators, AKI treated with renal replacement therapy in critically Ill patients with COVID-19, J. Am. Soc. Nephrol, doi:10.1681/ASN.2020060897
Hickson, Langhi Prata, Bobart, Evans, Giorgadze et al., Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease, EBioMedicine, doi:10.1016/j.ebiom.2019.08.069
Hirsch, Ng, Ross, Sharma, Shah et al., Northwell COVID-19 research consortium, northwell nephrology COVID-19 research consortium, acute kidney injury in patients hospitalized with COVID-19, Kidney Int, doi:10.1016/j.kint.2020.05.006
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hori, Zamboni, Carrao, Goldman, Berretta, The inhibition of inflammasome by Brazilian propolis (EPP-AF), Evid. Based Complement. Altern. Med, doi:10.1155/2013/418508
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Ito, Chang, Wang, Park, Ikegaki et al., Anti-AIDS agents. 48.(1) Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis, J. Nat. Prod, doi:10.1021/np010211x
Keflie, Biesalski, Micronutrients and bioactive substances: their potential roles in combating COVID-19, Nutrition, doi:10.1016/j.nut.2020.111103
Kellum, Lameire, Aspelin, Barsoum, Burdmann et al., Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury, Kidney Int. Suppl, doi:10.1038/kisup.2012.1
Khayrani, Irdiani, Aditama, Pratami, Lischer et al., Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study, J. King Saud Univ. Sci, doi:10.1016/j.jksus.2020.101297
Kumar, Dhanjal, Kaul, Wadhwa, Sundar, Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1772108
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Lima, Brito, Da, Nizer, Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2), Phytother. Res, doi:10.1002/ptr.6872
Machado, Assunção, Da Silva, Reis, Costa et al., Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity, Evid. Based Complement. Altern. Med, doi:10.1155/2012/157652
Marquiafável, Nascimento, Barud, Marquele-Oliveira, De-Freitas et al., Development and characterization of a novel standardized propolis dry extract obtained by factorial design with high artepillin C content, J. Pharm. Technol. Drug Res, doi:10.7243/2050-120X-4-1
Maruta, He, PAK1-blockers: potential therapeutics against COVID-19, Med. Drug Discov, doi:10.1016/j.medidd.2020.100039
Maruta, Herbal therapeutics that block the oncogenic kinase PAK1: a practical approach towards PAK1-dependent diseases and longevity, Phytother. Res, doi:10.1002/ptr.5054
Miryan, Soleimani, Dehghani, Sohrabi, Khorvash et al., The effect of propolis supplementation on clinical symptoms in patients with coronavirus (COVID-19): A structured summary of a study protocol for a randomised controlled trial, Trials, doi:10.1186/s13063-020-04934-7
Oryan, Alemzadeh, Moshiri, Potential role of propolis in wound healing: biological properties and therapeutic activities, Biomed. Pharmacother, doi:10.1016/j.biopha.2017.12.069
Osés, Marcos, Azofra, De Pablo, Fernández-Muíño et al., Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: needs for analytical harmonization, Antioxidants, doi:10.3390/antiox9010075
Picolotto, Pergher, Pereira, Machado, Da Silva Barud et al., Bacterial cellulose membrane associated with red propolis as phytomodulator: Improved healing effects in experimental models of diabetes mellitus, Biomed. Pharmacother, doi:10.1016/j.biopha.2019.108640
Piñeros, De Lima, Rodrigues, Gembre, Bertolini et al., Green propolis increases myeloid suppressor cells and CD4(+)Foxp3(+) cells and reduces Th2 inflammation in the lungs after allergen exposure, J. Ethnopharmacol, doi:10.1016/j.jep.2019.112496
Ripari, Sartori, Honorio, Conte, Tasca et al., Propolis antiviral and immunomodulatory activity: a review and perspectives for COVID-19 treatment, J. Pharm. Pharmacol, doi:10.1093/jpp/rgaa067
Rocha, Bueno, Md, Vaz, Nascimento et al., Evaluation of a propolis water extract using a reliable RP-HPLC methodology and in vitro and in vivo efficacy and safety characterisation, Evid. Based Complement. Alternat. Med, doi:10.1155/2013/670451
Rodrigues, De Sá, Ishimoto, Becerra, Oliveira et al., Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J. Exp. Med, doi:10.1084/jem.20201707
Sawaya, Barbosa Da Silva Cunha, Marcucci, Analytical methods applied to diverse types of Brazilian propolis, Chem. Cent. J, doi:10.1186/1752-153X-5-27
Scorza, Gonçalves, Scorza, Fiorini, De Almeida et al., Propolis and coronavirus disease 2019 (COVID-19): lessons from nature, Complement. Ther. Clin. Pract, doi:10.1016/j.ctcp.2020.101227
Sforcin, Bankova, Propolis: is there a potential for the development of new drugs?, J. Ethnopharmacol, doi:10.1016/j.jep.2010.10.032
Sforcin, Propolis and the immune system: a review, J. Ethnopharmacol, doi:10.1016/j.jep.2007.05.012
Shimizu, Hino, Tsutsumi, Park, Watanabe et al., Antiinfluenza virus activity of propolis in vitro and its efficacy against influenza infection in mice, Antivir. Chem. Chemother, doi:10.1177/095632020801900102
Silveira, Capcha, Sanches, Moreira, Garnica et al., Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis, Sci. Rep, doi:10.1038/s41598-021-85124-6
Silveira, Teles, Berretta, Sanches, Rodrigues et al., Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebocontrolled trial, BMC Nephrol, doi:10.1186/s12882-019-1337–1337
Soleimani, Rezaie, Rajabzadeh, Gholizadeh Navashenaq, Abbaspour et al., Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: a randomized clinical trial, Phytother. Res, doi:10.1002/ptr.6937
Tao, Shen, Sun, Chen, Yan, Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy, Biomed. Pharmacother, doi:10.1016/j.biopha.2018.07.026
Tiveron, Rosalen, Franchin, Lacerda, Bueno-Silva et al., Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south brazilian organic propolis, PLoS One, doi:10.1371/journal.pone.0165588
Van Dam, Huizing, Mestach, Dierckxsens, Tjalma et al., SARS-CoV-2 and cancer: are they really partners in crime?, Cancer Treat. Rev, doi:10.1016/j.ctrv.2020.102068
Varga, Flammer, Steiger, Haberecker, Andermatt et al., Endothelial cell infection and endotheliitis in COVID-19, Lancet, doi:10.1016/S0140-6736(20)30937-5
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Woisky, Salatino, Analysis of propolis: some parameters and procedures for chemical quality control, J. Apic. Res, doi:10.1080/00218839.1998.11100961
Xue, Liu, Xu, Zhou, Ma et al., Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats, Biomed. Pharmacother, doi:10.1016/j.biopha.2019.109393
DOI record:
{
"DOI": "10.1016/j.biopha.2021.111526",
"ISSN": [
"0753-3322"
],
"URL": "http://dx.doi.org/10.1016/j.biopha.2021.111526",
"alternative-id": [
"S0753332221003115"
],
"article-number": "111526",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Biomedicine & Pharmacotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.biopha.2021.111526"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Masson SAS."
}
],
"author": [
{
"affiliation": [],
"family": "Silveira",
"given": "Marcelo Augusto Duarte",
"sequence": "first"
},
{
"affiliation": [],
"family": "De Jong",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berretta",
"given": "Andresa Aparecida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galvão",
"given": "Erica Batista dos Santos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ribeiro",
"given": "Juliana Caldas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cerqueira-Silva",
"given": "Thiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amorim",
"given": "Thais Chaves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conceição",
"given": "Luis Filipe Miranda Rebelo da",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomes",
"given": "Marcel Miranda Dantas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "Maurício Brito",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Souza",
"given": "Sergio Pinto de",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Marcele Helena Celestino Alves dos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "San Martin",
"given": "Raissa Lanna Araújo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Márcio de Oliveira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lírio",
"given": "Monique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Lis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sampaio",
"given": "Julio Cezar Miranda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendonça",
"given": "Renata",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ultchak",
"given": "Silviana Salles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amorim",
"given": "Fabio Santos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramos",
"given": "João Gabriel Rosa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Batista",
"given": "Paulo Benigno Pena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guarda",
"given": "Suzete Nascimento Farias da",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendes",
"given": "Ana Verena Almeida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Passos",
"given": "Rogerio da Hora",
"sequence": "additional"
}
],
"container-title": [
"Biomedicine & Pharmacotherapy"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"em-consulte.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
3,
20
]
],
"date-time": "2021-03-20T21:46:18Z",
"timestamp": 1616276778000
},
"deposited": {
"date-parts": [
[
2021,
6,
5
]
],
"date-time": "2021-06-05T18:42:28Z",
"timestamp": 1622918548000
},
"indexed": {
"date-parts": [
[
2022,
1,
7
]
],
"date-time": "2022-01-07T08:08:34Z",
"timestamp": 1641542914883
},
"is-referenced-by-count": 13,
"issn-type": [
{
"type": "print",
"value": "0753-3322"
}
],
"issued": {
"date-parts": [
[
2021,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
1
]
],
"date-time": "2021-06-01T00:00:00Z",
"timestamp": 1622505600000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
3,
18
]
],
"date-time": "2021-03-18T00:00:00Z",
"timestamp": 1616025600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0753332221003115?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0753332221003115?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "111526",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
6
]
]
},
"published-print": {
"date-parts": [
[
2021,
6
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.biopha.2021.111526_bib1",
"unstructured": "Johns Hopkins University & Medicine. Coronavirus Resource Center, https://coronavirus.jhu.edu/, 2021 (Accessed 20 February 2021)."
},
{
"DOI": "10.1001/jama.2020.19759",
"article-title": "The COVID-19 pandemic and the $16 Trillion Virus",
"author": "Cutler",
"doi-asserted-by": "crossref",
"first-page": "1495",
"journal-title": "JAMA",
"key": "10.1016/j.biopha.2021.111526_bib2",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/j.ctrv.2020.102068",
"article-title": "SARS-CoV-2 and cancer: are they really partners in crime?",
"author": "van Dam",
"doi-asserted-by": "crossref",
"journal-title": "Cancer Treat. Rev.",
"key": "10.1016/j.biopha.2021.111526_bib3",
"volume": "89",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "protein interaction map reveals targets for drug repurposing",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "10.1016/j.biopha.2021.111526_bib4",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1007/s40121-020-00349-8",
"article-title": "Early treatment of COVID-19 disease: a missed opportunity",
"author": "Forrest",
"doi-asserted-by": "crossref",
"first-page": "715",
"journal-title": "Infect. Dis. Ther.",
"key": "10.1016/j.biopha.2021.111526_bib5",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.biopha.2021.111526_bib6",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2180-5",
"article-title": "Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Nature",
"key": "10.1016/j.biopha.2021.111526_bib7",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1016/j.medidd.2020.100039",
"article-title": "PAK1-blockers: potential therapeutics against COVID-19",
"author": "Maruta",
"doi-asserted-by": "crossref",
"journal-title": "Med. Drug Discov.",
"key": "10.1016/j.biopha.2021.111526_bib8",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2020.110622",
"article-title": "Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease",
"author": "Berretta",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib9",
"volume": "131",
"year": "2020"
},
{
"DOI": "10.1002/ptr.5054",
"article-title": "Herbal therapeutics that block the oncogenic kinase PAK1: a practical approach towards PAK1-dependent diseases and longevity",
"author": "Maruta",
"doi-asserted-by": "crossref",
"first-page": "656",
"journal-title": "Phytother. Res.",
"key": "10.1016/j.biopha.2021.111526_bib10",
"volume": "28",
"year": "2014"
},
{
"DOI": "10.1084/jem.20201707",
"article-title": "Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients",
"author": "Rodrigues",
"doi-asserted-by": "crossref",
"journal-title": "J. Exp. Med.",
"key": "10.1016/j.biopha.2021.111526_bib11",
"volume": "218",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/j.biopha.2021.111526_bib12",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1155/2019/1907698",
"article-title": "Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer",
"author": "Da",
"doi-asserted-by": "crossref",
"journal-title": "Anal. Cell. Pathol.",
"key": "10.1016/j.biopha.2021.111526_bib13",
"volume": "2019",
"year": "2019"
},
{
"DOI": "10.3390/foods7030041",
"article-title": "Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products",
"author": "Galeotti",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "3",
"journal-title": "Foods",
"key": "10.1016/j.biopha.2021.111526_bib14",
"volume": "7",
"year": "2018"
},
{
"article-title": "Functional properties of brazilian propolis: from chemical composition until the market",
"author": "Berretta",
"first-page": "55",
"key": "10.1016/j.biopha.2021.111526_bib15",
"series-title": "Superfood and Functional Food - An Overview of Their Processing and Utilization",
"year": "2017"
},
{
"DOI": "10.3390/antiox9010075",
"article-title": "Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: needs for analytical harmonization",
"author": "Osés",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Antioxidants",
"key": "10.1016/j.biopha.2021.111526_bib16",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1155/2012/157652",
"article-title": "Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity",
"author": "Machado",
"doi-asserted-by": "crossref",
"journal-title": "Evid. Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.111526_bib17",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1093/jpp/rgaa067",
"article-title": "Propolis antiviral and immunomodulatory activity: a review and perspectives for COVID-19 treatment",
"author": "Ripari",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "J. Pharm. Pharmacol.",
"key": "10.1016/j.biopha.2021.111526_bib18",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1177/095632020801900102",
"article-title": "Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice",
"author": "Shimizu",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Antivir. Chem. Chemother.",
"key": "10.1016/j.biopha.2021.111526_bib19",
"volume": "19",
"year": "2008"
},
{
"DOI": "10.1021/np010211x",
"article-title": "Anti-AIDS agents. 48.(1) Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis",
"author": "Ito",
"doi-asserted-by": "crossref",
"first-page": "1278",
"journal-title": "J. Nat. Prod.",
"key": "10.1016/j.biopha.2021.111526_bib20",
"volume": "64",
"year": "2001"
},
{
"DOI": "10.1016/j.jep.2005.05.004",
"article-title": "Chemical diversity of propolis and the problem of standardization",
"author": "Bankova",
"doi-asserted-by": "crossref",
"first-page": "114",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.111526_bib21",
"volume": "100",
"year": "2005"
},
{
"DOI": "10.1186/1752-153X-5-27",
"article-title": "Analytical methods applied to diverse types of Brazilian propolis",
"author": "Sawaya",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "Chem. Cent. J.",
"key": "10.1016/j.biopha.2021.111526_bib22",
"volume": "5",
"year": "2011"
},
{
"DOI": "10.1007/s12016-012-8322-2",
"article-title": "The immunomodulatory and anticancer properties of propolis",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "262",
"journal-title": "Clin. Rev. Allerg. Immunol.",
"key": "10.1016/j.biopha.2021.111526_bib23",
"volume": "44",
"year": "2013"
},
{
"DOI": "10.1016/j.jep.2010.10.032",
"article-title": "Propolis: is there a potential for the development of new drugs?",
"author": "Sforcin",
"doi-asserted-by": "crossref",
"first-page": "253",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.111526_bib24",
"volume": "133",
"year": "2011"
},
{
"DOI": "10.7150/ijbs.3641",
"article-title": "Propolis standardized extract (EPP-AF(R)), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds",
"author": "Berretta",
"doi-asserted-by": "crossref",
"first-page": "512",
"journal-title": "Int. J. Biol. Sci.",
"key": "10.1016/j.biopha.2021.111526_bib25",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.7243/2050-120X-4-1",
"article-title": "Development and characterization of a novel standardized propolis dry extract obtained by factorial design with high artepillin C content",
"author": "Marquiafável",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Pharm. Technol. Drug Res.",
"key": "10.1016/j.biopha.2021.111526_bib26",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1016/j.jep.2019.112174",
"article-title": "Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF®) using an in vivo cocktail approach",
"author": "Cusinato",
"doi-asserted-by": "crossref",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.111526_bib27",
"volume": "245",
"year": "2019"
},
{
"DOI": "10.1186/s12882-019-1337-7",
"article-title": "Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial",
"author": "Silveira",
"doi-asserted-by": "crossref",
"first-page": "140",
"journal-title": "BMC Nephrol.",
"key": "10.1016/j.biopha.2021.111526_bib28",
"volume": "20",
"year": "2019"
},
{
"article-title": "Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: a randomized clinical trial",
"author": "Soleimani",
"journal-title": "Phytother. Res.",
"key": "10.1016/j.biopha.2021.111526_bib29",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/j.jep.2007.05.012",
"article-title": "Propolis and the immune system: a review",
"author": "Sforcin",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.111526_bib30",
"volume": "113",
"year": "2007"
},
{
"DOI": "10.1155/2013/418508",
"article-title": "The inhibition of inflammasome by Brazilian propolis (EPP-AF)",
"author": "Hori",
"doi-asserted-by": "crossref",
"journal-title": "Evid. Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.111526_bib31",
"volume": "2013",
"year": "2013"
},
{
"DOI": "10.1371/journal.pone.0165588",
"article-title": "Chemical characterization and antioxidant, antimicrobial, and anti-inflammatory activities of south brazilian organic propolis",
"author": "Tiveron",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "PLoS One",
"key": "10.1016/j.biopha.2021.111526_bib32",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.1016/j.biopha.2017.05.027",
"article-title": "Antitumor activity of Brazilian red propolis fractions against Hep-2 cancer cell line",
"author": "Frozza",
"doi-asserted-by": "crossref",
"first-page": "951",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib33",
"volume": "91",
"year": "2017"
},
{
"DOI": "10.1016/j.biopha.2017.12.069",
"article-title": "Potential role of propolis in wound healing: biological properties and therapeutic activities",
"author": "Oryan",
"doi-asserted-by": "crossref",
"first-page": "469",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib34",
"volume": "98",
"year": "2018"
},
{
"DOI": "10.1016/j.biopha.2018.07.026",
"article-title": "Neuroprotective effects of pinocembrin on ischemia/reperfusion-induced brain injury by inhibiting autophagy",
"author": "Tao",
"doi-asserted-by": "crossref",
"first-page": "1003",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib35",
"volume": "106",
"year": "2018"
},
{
"DOI": "10.1016/j.biopha.2019.108640",
"article-title": "Bacterial cellulose membrane associated with red propolis as phytomodulator: Improved healing effects in experimental models of diabetes mellitus",
"author": "Picolotto",
"doi-asserted-by": "crossref",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib36",
"volume": "112",
"year": "2019"
},
{
"DOI": "10.1016/j.biopha.2018.10.116",
"article-title": "Hydroalcoholic extract of Brazilian green propolis modulates inflammatory process in mice submitted to a low protein diet",
"author": "de Miranda",
"doi-asserted-by": "crossref",
"first-page": "610",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib37",
"volume": "109",
"year": "2019"
},
{
"DOI": "10.1155/2020/7538232",
"article-title": "Antioxidant effect of standardized extract of propolis (EPP-AF®) in healthy volunteers: a “before and after” clinical study",
"author": "Diniz",
"doi-asserted-by": "crossref",
"journal-title": "Evid. Based Complement. Altern. Med.",
"key": "10.1016/j.biopha.2021.111526_bib38",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/j.jep.2019.112496",
"article-title": "Green propolis increases myeloid suppressor cells and CD4(+)Foxp3(+) cells and reduces Th2 inflammation in the lungs after allergen exposure",
"author": "Piñeros",
"doi-asserted-by": "crossref",
"journal-title": "J. Ethnopharmacol.",
"key": "10.1016/j.biopha.2021.111526_bib39",
"volume": "252",
"year": "2020"
},
{
"DOI": "10.1016/j.sjbs.2020.12.031",
"article-title": "Fighting against the second wave of COVID-19: can honeybee products help protect against the pandemic?",
"author": "Al Naggar",
"doi-asserted-by": "crossref",
"first-page": "1519",
"journal-title": "Saudi J. Biol. Sci.",
"key": "10.1016/j.biopha.2021.111526_bib40",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1111/dth.13780",
"article-title": "Back to the basics: propolis and COVID-19",
"author": "Bachevski",
"doi-asserted-by": "crossref",
"journal-title": "Dermatol. Ther.",
"key": "10.1016/j.biopha.2021.111526_bib41",
"volume": "33",
"year": "2020"
},
{
"article-title": "Micronutrients and bioactive substances: their potential roles in combating COVID-19",
"author": "Keflie",
"journal-title": "Nutrition",
"key": "10.1016/j.biopha.2021.111526_bib42",
"volume": "84",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6872",
"article-title": "Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2)",
"author": "Lima",
"doi-asserted-by": "crossref",
"first-page": "743",
"journal-title": "Phytother. Res.",
"key": "10.1016/j.biopha.2021.111526_bib43",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1016/j.ctcp.2020.101227",
"article-title": "Propolis and coronavirus disease 2019 (COVID-19): lessons from nature",
"author": "Scorza",
"doi-asserted-by": "crossref",
"journal-title": "Complement. Ther. Clin. Pract.",
"key": "10.1016/j.biopha.2021.111526_bib44",
"volume": "41",
"year": "2020"
},
{
"article-title": "Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity",
"author": "Kumar",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.biopha.2021.111526_bib45",
"year": "2020"
},
{
"DOI": "10.1080/00218839.1998.11100961",
"article-title": "Analysis of propolis: some parameters and procedures for chemical quality control",
"author": "Woisky",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "J. Apic. Res.",
"key": "10.1016/j.biopha.2021.111526_bib46",
"volume": "37",
"year": "1998"
},
{
"DOI": "10.1155/2013/670451",
"article-title": "Evaluation of a propolis water extract using a reliable RP-HPLC methodology and in vitro and in vivo efficacy and safety characterisation",
"author": "Rocha",
"doi-asserted-by": "crossref",
"journal-title": "Evid. Based Complement. Alternat. Med.",
"key": "10.1016/j.biopha.2021.111526_bib47",
"volume": "2013",
"year": "2013"
},
{
"article-title": "Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury",
"author": "Kellum",
"first-page": "1",
"journal-title": "Kidney Int. Suppl.",
"key": "10.1016/j.biopha.2021.111526_bib48",
"volume": "2",
"year": "2012"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "New Engl. J. Med.",
"key": "10.1016/j.biopha.2021.111526_bib49",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "New Engl. J. Med.",
"key": "10.1016/j.biopha.2021.111526_bib50",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "JAMA",
"key": "10.1016/j.biopha.2021.111526_bib51",
"volume": "323",
"year": "2020"
},
{
"article-title": "Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats",
"author": "Xue",
"issue": "2019",
"journal-title": "Biomed. Pharmacother.",
"key": "10.1016/j.biopha.2021.111526_bib52",
"volume": "118",
"year": "2019"
},
{
"DOI": "10.1186/s13063-020-04934-7",
"article-title": "The effect of propolis supplementation on clinical symptoms in patients with coronavirus (COVID-19): A structured summary of a study protocol for a randomised controlled trial",
"author": "Miryan",
"doi-asserted-by": "crossref",
"first-page": "996",
"journal-title": "Trials",
"key": "10.1016/j.biopha.2021.111526_bib53",
"volume": "21",
"year": "2020"
},
{
"article-title": "Potential anti-COVID-19 activity of Egyptian propolis using computational modeling",
"author": "Elwakil",
"journal-title": "Future Virol.",
"key": "10.1016/j.biopha.2021.111526_bib54",
"year": "2021"
},
{
"DOI": "10.1016/j.jksus.2020.101297",
"article-title": "Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study",
"author": "Khayrani",
"doi-asserted-by": "crossref",
"journal-title": "J. King Saud Univ. Sci.",
"key": "10.1016/j.biopha.2021.111526_bib55",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2019.08.069",
"article-title": "Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease",
"author": "Hickson",
"doi-asserted-by": "crossref",
"first-page": "446",
"journal-title": "EBioMedicine",
"key": "10.1016/j.biopha.2021.111526_bib56",
"volume": "47",
"year": "2019"
},
{
"DOI": "10.1080/0886022X.2020.1756323",
"article-title": "Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "393",
"journal-title": "Ren. Fail.",
"key": "10.1016/j.biopha.2021.111526_bib57",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1016/j.kint.2020.05.006",
"article-title": "Northwell COVID-19 research consortium, northwell nephrology COVID-19 research consortium, acute kidney injury in patients hospitalized with COVID-19",
"author": "Hirsch",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Kidney Int.",
"key": "10.1016/j.biopha.2021.111526_bib58",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1681/ASN.2020040419",
"article-title": "COVID-19 and ACE2 in Cardiovascular, lung, and kidney working group. acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology",
"author": "Batlle",
"doi-asserted-by": "crossref",
"first-page": "1380",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "10.1016/j.biopha.2021.111526_bib59",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1681/ASN.2020060897",
"article-title": "AKI treated with renal replacement therapy in critically Ill patients with COVID-19",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "10.1016/j.biopha.2021.111526_bib60",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"article-title": "Endothelial cell infection and endotheliitis in COVID-19",
"author": "Varga",
"doi-asserted-by": "crossref",
"first-page": "1417",
"journal-title": "Lancet",
"key": "10.1016/j.biopha.2021.111526_bib61",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-85124-6",
"article-title": "Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis",
"author": "Silveira",
"doi-asserted-by": "crossref",
"first-page": "5925",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.biopha.2021.111526_bib62",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.phymed.2020.153368",
"article-title": "A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): a monocentric, randomized, double-blind, placebo-controlled clinical trial",
"author": "Esposito",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.biopha.2021.111526_bib63",
"volume": "80",
"year": "2021"
},
{
"DOI": "10.6061/clinics/2021/e2357",
"article-title": "Antiviral activity of Brazilian green propolis extract against SARS-CoV-2 (severe acute respiratory syndrome - coronavirus 2) infection: case report and review",
"author": "Fiorini",
"doi-asserted-by": "crossref",
"journal-title": "Clinics",
"key": "10.1016/j.biopha.2021.111526_bib64",
"volume": "76",
"year": "2021"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"score": 1,
"short-container-title": [
"Biomedicine & Pharmacotherapy"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": [
"Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "138"
}