Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study
et al., Medical Research Archives, doi:10.18103/mra.v11i2.3364, NCT04463264, Mar 2021 (preprint)
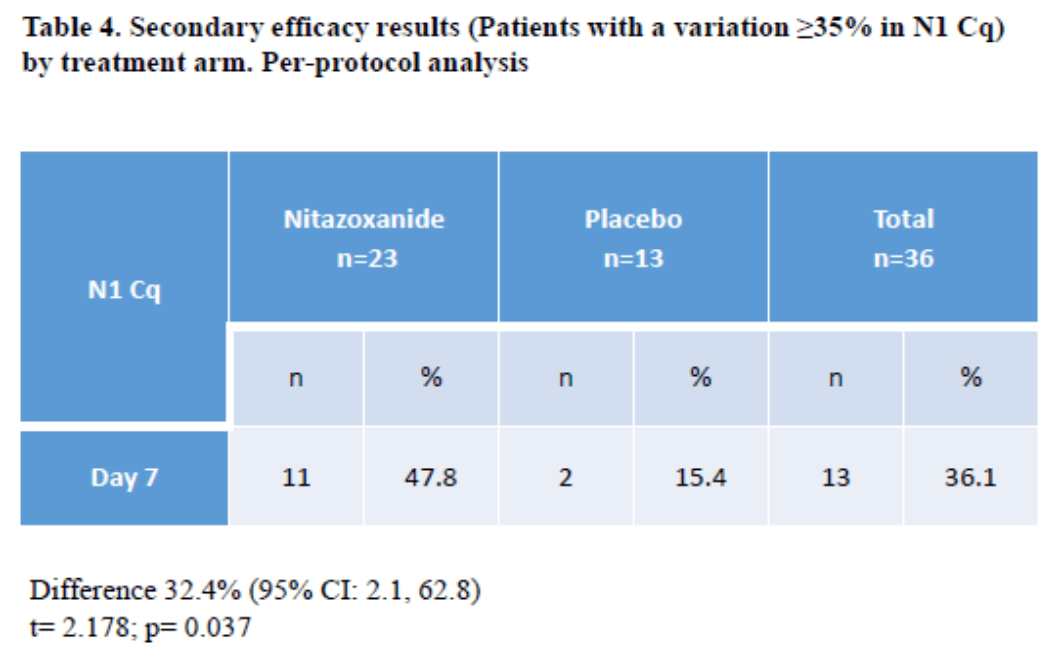
Small RCT with 23 nitazoxanide and 13 control patients showing significantly more patients achieved over 35% reduction in viral load from baseline.
|
relative mean improvement in Ct, 26.5% better, RR 0.74, p = 0.36, treatment 23, control 13.
|
|
risk of viral load reduction < 35% at day 7, 38.3% lower, RR 0.62, p = 0.08, treatment 12 of 23 (52.2%), control 11 of 13 (84.6%), NNT 3.1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Silva et al., 5 Mar 2021, Single Blind Randomized Controlled Trial, Argentina, peer-reviewed, 12 authors, study period July 2020 - December 2020, trial NCT04463264 (history).
Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study.
doi:10.1101/2021.03.03.21252509
The fast spread of COVID-19 has overcrowded Public Health Systems facilities in major countries due to the large number of seriously ill patients, particularly those requiring admission to intensive care units. Reducing viral load, along with other recommended epidemiological measures, such as social distancing and home confinement, can in time significantly help to reduce the infection R0 (Basic Reproductive Rate) and then mitigate disease burden. Early negativization or otherwise reduction of the viral load can potentially diminish disease severity, resulting in a better-controlled public health response, avoiding collapse of healthcare systems. Nitazoxanide, a widely used thiazolide approved by the FDA as an antiparasitic drug, also approved in Brazil for Norovirus and Rotavirus treatments, has an excellent safety record for a variety of indications. Nitazoxanide exhibits activity in vitro against MERS-CoV and other coronaviruses; and a specific antiviral effect (in micro molar doses) against SARS-CoV-2. The objective of this study was to evaluate the efficacy and safety of Nitazoxanide in reducing the SARS-COV 2 viral load within 7 days of treatment in respiratory samples from COVID-19-infected patients with mild to moderate disease, compared to placebo. An interim analysis showed that the ratio of patients with a viral load reduction ≥ 35% from baseline up to day 7 of treatment was significantly greater for Nitazoxanide compared to placebo (47.8% vs.
References
Ashiru, Howe, Butters, Nitazoxanide, an antiviral thiazolide, depletes ATP-sensitive intracelular Ca (2+) stores, Virology, doi:10.1016/j.virol.2014.05.015
Balderas-Acata, Bueno, Pérez-Becerril, Bioavailability of Two Oral-Suspension Formulations of a Single Dose of Nitazoxanide 500 mg: An Open-Label, Randomized-Sequence, Two-Period Crossover, Comparison in Healthy Fasted Mexican Adult Volunteers, Journal of Bioequivalence & Bioavailability
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19: final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Cascella, Rajnik, Cuomo, Evaluation and Treatment Coronavirus (COVID-19)
Chang, Mo, Yuan, Time Kinetics of Viral Clearance and Resolution of Symptoms in Novel Coronavirus Infection, Am J Respir Crit CareMed, doi:10.1164/rccm.202003-0524LE
Chen, Lau, Lamirande, Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection, J Virol, doi:10.1128/JVI.01281-0915
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Haffizulla, Hartman, Hoppers, Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo controlled, phase 2b/3trial, Lancet Infect Dis, doi:10.1016/S1473-3099(14)70717-0
Hong, Kim, Song, Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice, International Immunopharmacology, doi:10.1016/j.intimp.2012.03.002
Hui, Lee, Chan, Beigel, Cadena et al., The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus, Antiviral Res, doi:10.1016/j.antiviral.2018.01.002
Island, None
Kim, Read, Fauci, Therapy for Early COVID-19: A Critical Need, JAMA, doi:10.1001/jama.2020.22813
Liuboschitz, Abella, Jolkovsky, Biney, kyhlatamconsulting.com) and Andrea Federico (afederico@kyhlatamconsulting.com) from K&H Latam Consulting SAS. Statistical analysis and collaboration in the writing of the manuscript, doi:10.1001/jamainternmed.2020.6319
Lokhande, Devarajan, A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19, European Journal of Pharmacology, doi:10.1016/j.ejphar.2020.173748
Pujadas, Chaudhry, Mcbride, SARS-CoV-2 viral load predicts COVID-19 mortality, The Lancet Respiratory Medicine
Rajoli, Pertinez, Arshad, Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis, Br J Clin Pharmacol, doi:10.1111/bcp.14619
Riediker, Tsai, Estimation of Viral Aerosol Emissions From Simulated Individuals With Asymptomatic to Moderate Coronavirus Disease 2019, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13807
Rocco, Silva, Cruz, Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial, Eur Respir J, doi:10.1183/13993003.03725-2020
Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus, J Infect Public Health, doi:10.1016/j.jiph.2016.04.001
Rossignol, Nitazoxanide: a first-in-class broad-spectrum antiviral agent, Antiviral Res, doi:10.1016/j.antiviral.2014.07.014
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Zheng, Yu, Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study, BMJ, doi:10.1136/bmj.m1443
DOI record:
{
"DOI": "10.18103/mra.v11i2.3364",
"ISSN": [
"2375-1916",
"2375-1924"
],
"URL": "http://dx.doi.org/10.18103/mra.v11i2.3364",
"abstract": "<jats:p>The fast spread of COVID-19 has overcrowded Public Health Systems facilities in major countries due to the large number of seriously ill patients, particularly those requiring admission to intensive care units. Reducing viral load, along with other recommended epidemiological measures, such as social distancing and home confinement, can in time significantly help to reduce the infection R0 (Basic Reproductive Rate) and then mitigate disease burden. Early negativization or otherwise reduction of the viral load can potentially diminish disease severity, resulting in a better-controlled public health response, avoiding collapse of healthcare systems. Nitazoxanide, a widely used thiazolide approved by the FDA as an antiparasitic drug, also approved in Brazil for Norovirus and Rotavirus treatments, has an excellent safety record for a variety of indications. Nitazoxanide exhibits activity in vitro against MERS-CoV and other coronaviruses; and a specific antiviral effect (in micro molar doses) against SARS-CoV-2. The objective of this study was to evaluate the efficacy and safety of Nitazoxanide in reducing the SARS-COV 2 viral load within 7 days of treatment in respiratory samples from COVID-19-infected patients with mild to moderate disease, compared to placebo. An interim analysis showed that the ratio of patients with a viral load reduction ≥ 35% from baseline up to day 7 of treatment was significantly greater for Nitazoxanide compared to placebo (47.8% vs. 15.4%; Δ 34.6%; 95% CI: 64.7; 4.6: p = 0.037).</jats:p>",
"author": [
{
"affiliation": [],
"family": "Silva",
"given": "Marcelo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Espejo",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pereyra",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lynch",
"given": "Martín",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Marcos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laborde",
"given": "Luciana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taconelli",
"given": "Hernán",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patricia",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pereson",
"given": "Matías",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garbini",
"given": "Marcelo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crucci",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Enriquez",
"given": "Diego",
"sequence": "additional"
}
],
"container-title": "Medical Research Archives",
"container-title-short": "MRAJ",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
9
]
],
"date-time": "2023-04-09T05:18:01Z",
"timestamp": 1681017481000
},
"deposited": {
"date-parts": [
[
2023,
4,
9
]
],
"date-time": "2023-04-09T05:18:24Z",
"timestamp": 1681017504000
},
"indexed": {
"date-parts": [
[
2023,
4,
9
]
],
"date-time": "2023-04-09T05:41:24Z",
"timestamp": 1681018884283
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"member": "7483",
"original-title": [],
"prefix": "10.18103",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Knowledge Enterprise Journals",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://esmed.org/MRA/mra/article/view/3364"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Arts and Humanities"
],
"subtitle": [],
"title": "Efficacy of Nitazoxanide in reducing the viral ad in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel-group, pilot study.",
"type": "journal-article",
"volume": "11"
}