Potential of the Combination of a Systemic Enzyme Complex and Probiotics administration to Combat COVID-19: A Randomized Open Label Prospective Analysis
et al., Advances in Clinical Toxicology, doi:10.23880/act-16000204, Feb 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small RCT 60 patients in India, 30 treated with ImmunoSEB and ProbioSEB CSC3, showing faster recovery with treatment. CTRI/2020/09/027685, CTRI/2020/08/027168.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
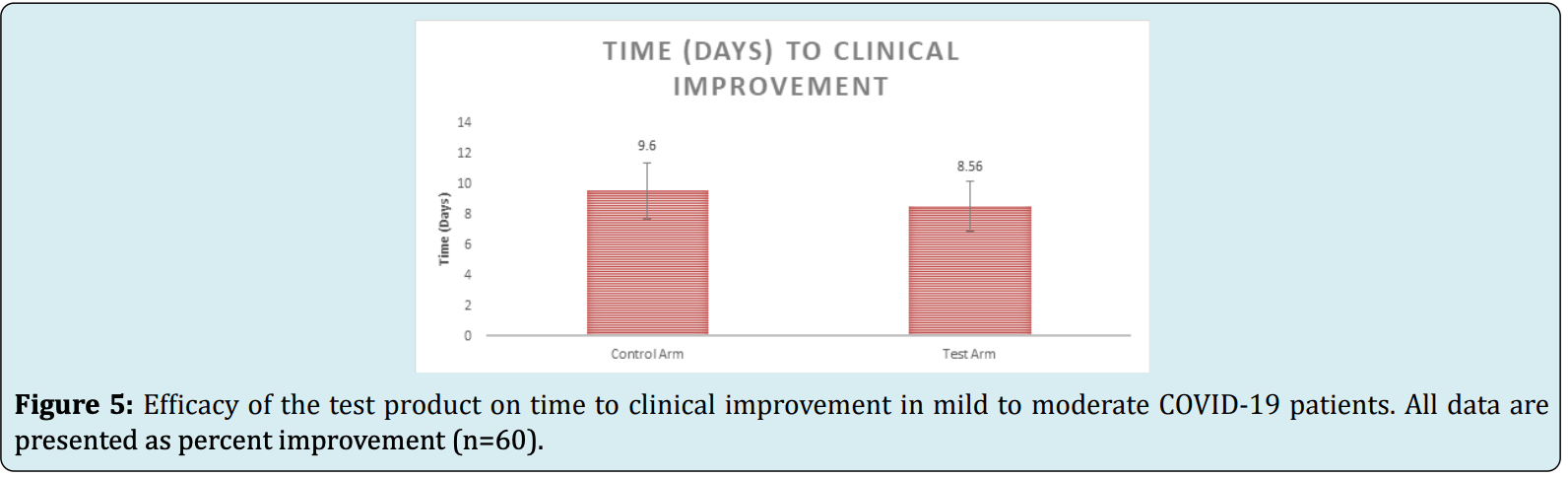
time to clinical improvement, 10.8% lower, relative time 0.89, p = 0.19, treatment 30, control 30.
|
|
hospitalization time, 10.6% lower, relative time 0.89, p = 0.18, treatment 30, control 30.
|
|
risk of no clinical improvement, 83.3% lower, RR 0.17, p = 0.005, treatment 2 of 30 (6.7%), control 12 of 30 (40.0%), NNT 3.0, day 10 mid-recovery.
|
|
risk of no clinical improvement, 3.7% lower, RR 0.96, p = 1.00, treatment 26 of 30 (86.7%), control 27 of 30 (90.0%), NNT 30, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shah et al., 2 Feb 2021, Randomized Controlled Trial, India, peer-reviewed, 3 authors, this trial uses multiple treatments in the treatment arm (combined with multi-enzyme formulation) - results of individual treatments may vary.
Potential of the Combination of a Systemic Enzyme Complex and Probiotics administration to Combat COVID-19: A Randomized Open Label Prospective Analysis
Advances in Clinical Toxicology, doi:10.23880/act-16000205
Background: Enzymes have been used for therapeutic applications for decades owing to their anti-inflammatory and immunomodulatory effects. Probiotics are well known to reduce the incidence and severity of several health-related conditions. To our knowledge, no clinical trial has evaluated the effects of a combination of systemic enzyme and probiotic supplementation in Covid-19 patients infected with the SARSCoV-2 virus. Objective: We investigated the safety and efficacy of the health supplements ImmunoSEB (systemic enzyme complex) and ProbioSEB CSC3 (probiotic complex) as supplemental therapy in confirmed mild to moderate COVID-19 patients.
Methods: A randomized, open label, 2-arm, prospective study in patients with an RT-PCR confirmed diagnosis of COVID-19 with a mild to moderate condition was conducted. The control arm (n=30) received standard of care (SOC) treatment and the test arm (n=30) received the oral supplements ImmunoSEB (500 mg/cap.) + ProbioSEB CSC3 (5 billion CFUs /cap.) for 14 days in addition to SOC. The efficacy and safety of the experimental regimen was compared with the control arm at various timepoints from days 1 to 21. Results: A significantly higher proportion of patients in the test arm showed clinical improvement on day 10 vs the controls (93.33% vs 60%; p<0.05). No adverse events were reported in the test arm at any time during the study suggesting the safety of supplementation with ImmunoSEB + ProbioSEB CSC3. Patients in the test arm also had a shorter duration of hospitalization, quicker recovery and faster reduction in CRP levels as compared to the control arm.
Conclusions: The present study concludes that supplemental therapy with ImmunoSEB + ProbioSEB CSC3 accelerates clinical improvement in mild to moderate COVID-19 patients. While there is no vaccine or specific drug to completely cure SARS CoV-2 infection, the proposed supplemental therapy could be a potential tool to aid in the recovery of COVID-19 of patients.
Author's contribution Dr. Rohit Parate and Dr. Abhay Vispute acted as principal investigators of this study and Dr Neha Shah was involved in study design, planning, protocol writing, data analysis and manuscript preparation.
References
Baron, A patented strain of Bacillus coagulans increased immune response to viral challenge, Postgrad Med
Blaabjerg, Artzi, Aabenhus, Blaabjerg, Probiotics for the prevention of antibiotic-associated diarrhea in outpatients-A systematic review and metaanalysis, Antibiotic
Cook, Johnstone, Marshall, Lauzier, Thabane, Probiotics: prevention of severe pneumonia and endotracheal colonization Trial-prospect: a pilot trial, Trials
Craik, Page, Proteases as therapeutics, Biochem J
Elshaghabee, Rokana, Gulhane, Sharma, Panwar, Bacillus as potential probiotics: status, concerns, and future perspectives, Front Microbiol, doi:10.3389/fmicb.2017.01490/full
Fadl, Ahmed, Booles, Sayed, Serrapeptase and nattokinase intervention for relieving Alzheimer's disease pathophysiology in rat model, Hum Exp Toxicol
Farhadi, Bracho-Sanchez, Freeman, Keselowsky, Hudalla, Enzymes as immunotherapeutics, Bioconjug Chem
Gioia, Ciaccio, Calligari, Simone, Sbardella, Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches, Biochem Pharmacol
Jadhav, Shah, Rathi, Rathi, Rathi, Serratiopeptidase: Insights into the therapeutic applications, Biotechnol Rep
Johnstone, Heels-Ansdell, Thabane, Meade, Marshall, Evaluating probiotics for the prevention of ventilator-associated pneumonia: a randomised placebo-controlled multicentre trial protocol and statistical analysis plan for Prospect, BMJ Open
Kassa, Antiviral probiotics: A new concept in medical sciences, New Insights on Antiviral Probiotics, doi:10.1007/978-3-319-49688-7_1
Lefevre, Racedo, Ripert, Housez, Cazaubiel, Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: a randomized, double-blind placebo-controlled study, Immun Ageing
Liu, Alookaran, Rhoads, Probiotics in autoimmune and inflammatory disorders, Nutrients
Mann, Ndung, The potential of lactoferrin, ovotransferrin and lysozyme as antiviral and immunemodulating agents in COVID-19, Future Virol, doi:10.2217/fvl-2020-0170
Marseglia, Tosca, Cirillo, Licari, Leone, Efficacy of Bacillus clausii spores in the prevention of recurrent respiratory infections in children: a pilot study, Ther Clin Risk Manag
Marzin, Lorkowski, Reule, Rau, Pabst, Effects of a systemic enzyme therapy in healthy active adults after exhaustive eccentric exercise: a randomised, two-stage, double-blinded, placebo-controlled trial, BMJ Open Sport Exerc Med
Mayo Clinic, COVID-19 (coronavirus): Long-term effects
Mazidi, Rezaie, Ferns, Vatanparast, Impact of probiotic administration on serum C -reactive protein concentrations: Systematic review and metaanalysis of randomized control trials, Nutrients
Małaczewska, Kaczorek-Łukowska, Wojcik, Siwicki, Antiviral effects of nisin, lysozyme, lactoferrin and their mixtures against bovine viral diarrhoea virus, BMC Vet Res, doi:10.1186/s12917-019-2067-6
Mohanty, Bendre, Sarangdhar, Fadia, Efficacy of lysozyme-lactoferrin as a bioenhancer (immunomodulator) in the treatment of Tuberculosis, RGUHS J Med Sci
Olaimat, Aolymat, Al-Holy, Ayyash, Ghoush, The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19
Pandey, Cabot, Shaw, Hewavitharana, Anti-inflammatory and immunomodulatory properties of Carica papaya, J Immunotoxicol
Qin, Cao, Wen, Yu, Liu, An antioxidant enzyme therapeutic for COVID-19, Adv Mater, doi:10.1002/adma.202004901
Rajinikanth, Venkatachalam, Manavalan, Investigations on the potential of serratiopeptidase-a proteolytic enzyme, on acetic acid induced ulcerative colitis in mice, Int J Pharm Pharm Sci
Rani, Immunomodulatory effects of proteolytic enzymes: meteoric brief review, Adv tissue Eng Regen Med
Scavone, Brusco, Mbertini, Sportiello, Rafaniello, Current pharmacological treatments for COVID-19: What's next?, Br J Pharmacol
Shah, Potential of the Combination of a Systemic Enzyme Complex and Probiotics administration to Combat COVID-19: A Randomized Open Label Prospective Analysis, Adv Clin Toxicol
Tiwari, The role of serratiopeptidase in the resolution of inflammation, Asian J Pharm Sci
DOI record:
{
"DOI": "10.23880/act-16000204",
"ISSN": [
"2577-4328"
],
"URL": "http://dx.doi.org/10.23880/act-16000204",
"abstract": "<jats:p>The pandemic-COVID-19 adverse effects on the environment, travel, education and clinical research, and the global health of humans and animals, and the impact on human-civilization, agriculture, global socio-economy, and damage brain-tissue or long term neurological-disorder, with more than 88-million infections and more than 1.8-million death of human lives. Recently the application of vaccine starts, but its proper efficacy, longevity, cost-effectiveness, allergic-toxic-reaction, and chance of reinfection due to new variant and mutation, are not still known. Though the middle-and upper-classes are able to tackle, but the economically poor-households, the marginalized in the Purba Bardhaman district, and groups like senior-citizens and street-children and animals, are badly affected still now. So in this paper students act as a 21st-century preventive-pandemicCOVID-19 model, improving advanced-clinical-toxicology, biomedicines, green-socio-economy, and science-technologyinnovations-communication by boosting community immunity or herd-immunity, and developing policy-initiative social strategies issues, removing the adverse effects of chemicals on living organisms and clinical studies in all areas of toxicology. This paper is considered the possible pathway of future pandemic COVID-19 like’s virus-free world.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Datta",
"given": "Subhas Chandra",
"sequence": "first"
}
],
"container-title": "Advances in Clinical Toxicology",
"container-title-short": "ACT",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T05:14:13Z",
"timestamp": 1648790053000
},
"deposited": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T05:14:15Z",
"timestamp": 1648790055000
},
"indexed": {
"date-parts": [
[
2023,
1,
27
]
],
"date-time": "2023-01-27T21:15:37Z",
"timestamp": 1674854137682
},
"is-referenced-by-count": 5,
"issue": "1",
"issued": {
"date-parts": [
[
2021
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2021
]
]
}
},
"member": "9969",
"original-title": [
"Students Act as 21st Century Preventive-Pandemic-COVID-19 Model: Improved Advance-Clinical-Toxicology Biomedicine Green-Socio-Economy Science-Technology-Innovations"
],
"prefix": "10.23880",
"published": {
"date-parts": [
[
2021
]
]
},
"published-online": {
"date-parts": [
[
2021
]
]
},
"publisher": "Medwin Publishers",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://medwinpublishers.com/ACT/Students%20Act%20as%2021st%20Century%20Preventive%20Pandemic%20COVID%2019%20Model%20Improved%20Advance%20Clinical%20Toxicology%20Biomedicine%20Green%20Socio%20Economy%20Science%20Technology%20Innovations.pdf"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Students Act as 21st Century Preventive-Pandemic-COVID-19 Model: Improved Advance-Clinical-Toxicology Biomedicine Green-Socio-Economy Science-Technology-Innovations",
"type": "journal-article",
"volume": "6"
}
