Efficacy and safety of adding fluoxetine to the treatment regimen of hospitalized patients with non‐critical COVID‐19 pneumonia: A double‐blind randomized, placebo‐controlled clinical trial
et al., Neuropsychopharmacology Reports, doi:10.1002/npr2.12327, Mar 2023
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
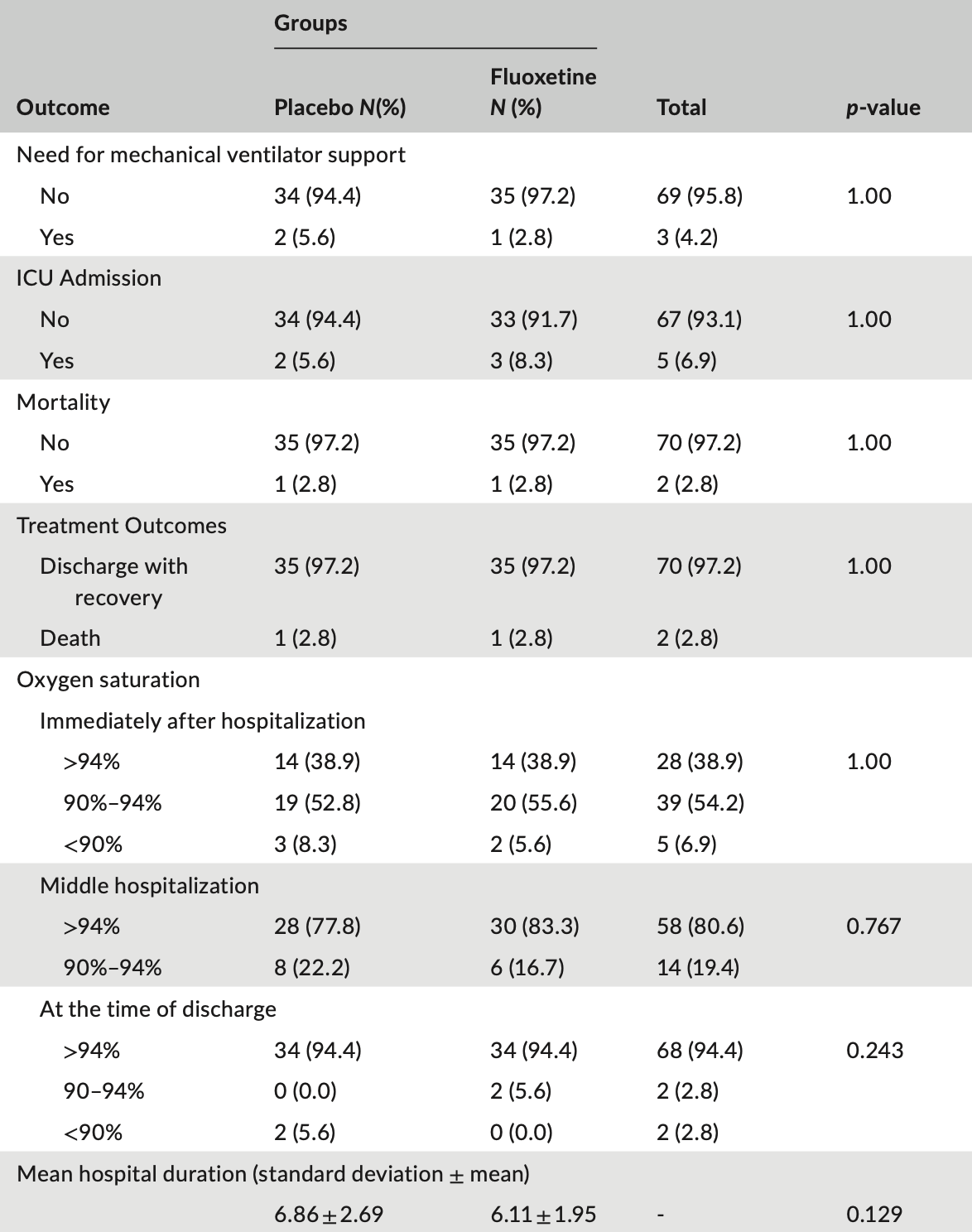
RCT 72 patients in Iran, showing faster reduction of inflammation with treatment. There was no significant difference in mortality, ventilation, or ICU admission (few events).
|
risk of death, no change, RR 1.00, p = 1.00, treatment 1 of 36 (2.8%), control 1 of 36 (2.8%).
|
|
risk of mechanical ventilation, 50.0% lower, RR 0.50, p = 1.00, treatment 1 of 36 (2.8%), control 2 of 36 (5.6%), NNT 36.
|
|
risk of ICU admission, 50.0% higher, RR 1.50, p = 1.00, treatment 3 of 36 (8.3%), control 2 of 36 (5.6%).
|
|
hospitalization time, 10.9% lower, relative time 0.89, p = 0.13, treatment 36, control 36.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sedighi et al., 20 Mar 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 8 authors.
Contact: forouzan.elyasi@gmail.com, f.elyasi@mazums.ac.ir, lab2002b@yahoo.com.
Efficacy and safety of adding fluoxetine to the treatment regimen of hospitalized patients with non‐critical COVID ‐19 pneumonia: A double‐blind randomized, placebo‐controlled clinical trial
Neuropsychopharmacology Reports, doi:10.1002/npr2.12327
Introduction: Selective serotonin reuptake inhibitors are considered the drugs, whose effectiveness in viral pandemics has been studied. The aim of this study was to evaluate of adding fluoxetine to the treatment regimen of patients with COVID-19 pneumonia.
Methods: This study was a double-blind randomized placebo controlled clinical trial .36 patients in the fluoxetine and 36 patients in the placebo group were enrolled. Patients in the intervention group were first treated with fluoxetine 10 mg for 4 days and then the dose of 20 mg was continued for 4 weeks. Data analysis was conducted using SPSS V. 22.0. Results: There was no statistically significant difference between the two groups in terms of clinical symptoms at the beginning of the study and also the score of anxiety and depression, oxygen saturation at the time of hospitalization, mid-hospitalization and discharge periods. The need for mechanical ventilator support (p = 1.00), the need for admission in the intensive care unit (ICU) (p = 1.00), rate for mortality (p = 1.00), and discharge with relative recovery (p = 1.00) were not significantly different between the two groups. The distribution of CRP within the study groups showed a significant decrease during different time periods (p = 0.001), and although there was no and at discharge (p = 0.585), mid-hospital CRP showed a significant decrease in the fluoxetine group (p = 0.032).
Conclusion: Fluoxetine resulted in a faster reduction of patients' inflammation without association with depression and anxiety.
reported the side effects of fluoxetine and the evidence of reduced inflammatory processes was observed in the fluoxetine group. It is suggested that in future studies, the sample size would be calculated with the higher power to examine whether stronger findings can be achieved with larger sample size. Also the dose of the drug could be increased more rapidly and, if possible, increasing the dose to higher than 20 mg per day, can lead to greater efficacy of the drug.
| CON CLUS ION
ACK N OWLED G M ENTS The study was done with the financial support from the Vice Chancellor for Research of MAZUMS. We thank all those who helped us in this study. We also thank the patients for their cooperation in the fulfillment of this study and all hospital staff involved in patient treatment.
FU N D I N G I N FO R M ATI O N This study was supported by the Mazandaran University of Medical Sciences. (Grant number = 8354).
CO N FLI C T O F I NTER E S T S TATEM ENT The authors declare that they have no conflict of interest.
References
Ahmadi Livani, Gohardehi, Azizi, Hashemvarzi, Taghavi et al., Multisectoral actions of mental health during the COVID-19 pandemic in Mazandaran province of Iran, Neuropsychopharmacol Rep, doi:10.1002/npr2.12239
Azizi, Elyasi, Roodposhti, Bradycardia caused by interaction of venlafaxine and cyclosporine: a case report, Caspian J Intern Med
Baller, Hogan, Fusunyan, Ivkovic, Luccarelli et al., Neurocovid: pharmacological recommendations for delirium associated with COVID-19, Psychosomatics, doi:10.1016/Fj.psym.2020.05.013
Bishara, Kalafatis, Taylor, Emerging and experimental treatments for COVID-19 and drug interactions with psychotropic agents, Ther Adv Psychopharmacol, doi:10.1177/F2045125320935306
Boland, Verduin, Ruiz, Antidepressants
Caiaffo, Oliveira, De Sá, Neto, Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine, Pharmacol Res Perspect, doi:10.1002/prp1002.1231
Carpinteiro, Edwards, Hoffmann, Kochs, Gripp et al., Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells, Cell Rep Med, doi:10.1002/prp1002.1231
Castro, Garcez, Silva, Patient care during the COVID-19 pandemic: do not leave delirium behind, Braz J Psychiatry, doi:10.1590/F1516-4446-2020-1048
Fda, Flouxetine, None
Gulbins, Palmada, Reichel, Lüth, Böhmer et al., Acid sphingomyelinase-ceramide system mediates effects of antidepressant drugs, Nat Med, doi:10.1038/nm.3214
Hiles, Baker, De Malmanche, Attia, Interleukin-6, C-reactive protein and interleukin-10 after antidepressant treatment in people with depression: a meta-analysis, Psychol Med, doi:10.1017/s0033291712000128
Hoertel, Rico, Vernet, Beeker, Jannot et al., Association between SSRI antidepressant use and reduced risk of intubation or death in hospitalized patients with coronavirus disease 2019: a multicenter retrospective observational study, doi:10.1038/s41380-41021-01021-41384
Kamali, Moosazadeh, Azizi, Ghasemian, Reskati et al., Anxiety due to COVID-19 among healthcare providers during pandemic: a web-based cross-sectional survey in Iran, Neuropsychopharmacol Rep, doi:10.1002/npr2.12213
Karimi-Khouzani, Heidarian, Amini, Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats, Pharmacol Rep, doi:10.1016/j.pharep.2017.1003.1011
Köhler, Freitas, Stubbs, Maes, Solmi et al., Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis, Mol Neurobiol, doi:10.1001/jama.2020.22760
Lenze, Mattar, Zorumski, Stevens, Schweiger et al., Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.22760
Montazeri, Vahdaninia, Ebrahimi, Jarvandi, The hospital anxiety and depression scale (HADS): translation and validation study of the Iranian version, Health Qual Life Outcomes, doi:10.1186/1477-7525-1181-1114
Németh, Szûcs, Vitrai, Juhász, Németh et al., Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: a retrospective case-control study, Ideggyogy Sz, doi:10.18071/isz.18074.10389
Oskotsky, Marić, Tang, Oskotsky, Wong et al., Efficacy and safety of adding fluoxetine to the treatment regimen of hospitalized patients with non-critical COVID-19 pneumonia: A double-blind randomized, placebo-controlled clinical trial, Neuropsychopharmacol Rep
Reis, Moreira-Silva, Silva, Thabane, Milagres et al., Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial, Lancet Glob Health, doi:10.1016/s2214-1109x(1021)00448-00444
Roumestan, Michel, Bichon, Portet, Detoc et al., Anti-inflammatory properties of desipramine and fluoxetine, Respir Res, doi:10.1186/1465-9921-1188-1135
Schloer, Brunotte, Goretzko, Mecate-Zambrano, Korthals et al., Targeting the endolysosomal host-SARS-CoV-2 interface by the clinically licensed antidepressant fluoxetine, Biorxiv, doi:10.1080/22221751.2020.1829082
Terauchi, Hiramitsu, Akiyoshi, Owa, Kato et al., Associations between anxiety, depression and insomnia in peri-and post-menopausal women, Maturitas, doi:10.1016/j.maturitas.2012.1001.1014
Williamson, Walker, Bhaskaran, Bacon, Bates et al., OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients, medRxiv, doi:10.1038/s41586-020-2521-4
Yang, Ding, Hu, Zhang, Sheng, Reliability and validity of a Chinese version of the HADS for screening depression and anxiety in psycho-cardiological outpatients, Compr Psychiatry, doi:10.1016/j.comppsych.2013.1008.1012
Zigmond, Snaith, The hospital anxiety and depression scale, Acta Psychiatr Scand, doi:10.1111/j.1600-0447.1983.tb09716.x
Zimniak, Kirschner, Hilpert, Geiger, Danov et al., The serotonin reuptake inhibitor fluoxetine inhibits SARS-CoV-2 in human lung tissue, Sci Rep, doi:10.1038/s41598-021-85049-0
DOI record:
{
"DOI": "10.1002/npr2.12327",
"ISSN": [
"2574-173X",
"2574-173X"
],
"URL": "http://dx.doi.org/10.1002/npr2.12327",
"alternative-id": [
"10.1002/npr2.12327"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-12-01"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-02-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-03-20"
}
],
"author": [
{
"affiliation": [
{
"name": "Psychiatry and Behavioral Sciences Research Center Addiction Institute Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Sedighi",
"given": "Faranak",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Psychiatry and Behavioral Sciences Research Center Addiction Institute Mazandaran University of Medical Sciences Sari Iran"
},
{
"name": "Department of Psychiatry Faculty of Medicine Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Zarghami",
"given": "Mehran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Psychiatry and Behavioral Sciences Research Center Addiction Institute Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Alizadeh Arimi",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gastrointestinal Cancer Research Center Non‐communicable Diseases Institute Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Moosazadeh",
"given": "Mahmood",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy Pharmaceutical Sciences Research Center Hemoglobinopathy Institute, Faculty of Pharmacy Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Ala",
"given": "Shahram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Antimicrobial Resistance Research Center Department of Infectious Diseases Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Ghasemian",
"given": "Roya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Pulmonary and Critical Care Division Faculty of Medicine Mazandaran University of Medical Sciences Sari Iran"
}
],
"family": "Mehravaran",
"given": "Hossein",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6872-481X",
"affiliation": [
{
"name": "Psychiatry and Behavioral Sciences Research Center Addiction Institute Mazandaran University of Medical Sciences Sari Iran"
},
{
"name": "Department of Psychiatry Faculty of Medicine Mazandaran University of Medical Sciences Sari Iran"
}
],
"authenticated-orcid": false,
"family": "Elyasi",
"given": "Forouzan",
"sequence": "additional"
}
],
"container-title": "Neuropsychopharmacology Reports",
"container-title-short": "Neuropsychopharm Rep",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T01:49:37Z",
"timestamp": 1679363377000
},
"deposited": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T01:49:51Z",
"timestamp": 1679363391000
},
"funder": [
{
"DOI": "10.13039/501100004160",
"award": [
"8354"
],
"doi-asserted-by": "publisher",
"name": "Mazandaran University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T05:18:37Z",
"timestamp": 1679375917275
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
3,
20
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
20
]
],
"date-time": "2023-03-20T00:00:00Z",
"timestamp": 1679270400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
20
]
],
"date-time": "2023-03-20T00:00:00Z",
"timestamp": 1679270400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/npr2.12327",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/npr2.12327",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/npr2.12327",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
3,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
20
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/npr2.12239",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_2_1"
},
{
"DOI": "10.1002/npr2.12213",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_3_1"
},
{
"DOI": "10.1016/Fj.psym.2020.05.013",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_4_1"
},
{
"DOI": "10.1590/F1516‐4446‐2020‐1048",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_5_1"
},
{
"DOI": "10.1080/22221751.2020.1829082",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_6_1"
},
{
"DOI": "10.1038/s41598‐021‐85049‐0",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_7_1"
},
{
"DOI": "10.1177/F2045125320935306",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_8_1"
},
{
"article-title": "Bradycardia caused by interaction of venlafaxine and cyclosporine: a case report",
"author": "Azizi M",
"first-page": "463",
"issue": "4",
"journal-title": "Caspian J Intern Med",
"key": "e_1_2_13_9_1",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1002/prp1002.1231",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_10_1"
},
{
"DOI": "10.1017/S0033291712000128",
"article-title": "Interleukin‐6, C‐reactive protein and interleukin‐ 10 after antidepressant treatment in people with depression: a meta‐analysis",
"author": "Hiles S",
"doi-asserted-by": "crossref",
"first-page": "2015",
"issue": "10",
"journal-title": "Psychol Med",
"key": "e_1_2_13_11_1",
"volume": "42",
"year": "2012"
},
{
"article-title": "Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta‐analysis",
"author": "Köhler CA",
"first-page": "4195",
"issue": "5",
"journal-title": "Mol Neurobiol",
"key": "e_1_2_13_12_1",
"volume": "55",
"year": "2018"
},
{
"key": "e_1_2_13_13_1",
"unstructured": "HoertelN RicoMS VernetR BeekerN JannotA‐S NeurazA et al.Association between SSRI antidepressant use and reduced risk of intubation or death in hospitalized patients with coronavirus disease 2019: a multicenter retrospective observational study.medRxiv.20205110.1038/s41380‐41021‐01021‐41384"
},
{
"key": "e_1_2_13_14_1",
"unstructured": "WilliamsonE WalkerAJ BhaskaranKJ BaconS BatesC MortonCE et al.OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients.medRxiv.2020https://doi.org/10.1038/s41586‐020‐2521‐4"
},
{
"DOI": "10.1186/1465‐9921‐1188‐1135",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_15_1"
},
{
"key": "e_1_2_13_16_1",
"unstructured": "FDA.flouxetine. Retrieved from2020https://wayback.archive‐it.org/7993/20170403214511/https://www.fda.gov/ucm/groups/fdagov‐public/@fdagov‐drugs‐gen/documents/document/ucm244383.pdf"
},
{
"DOI": "10.1111/j.1600‐0447.1983.tb09716.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_17_1"
},
{
"DOI": "10.1016/j.maturitas.2012.1001.1014",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_18_1"
},
{
"DOI": "10.1186/1477‐7525‐1181‐1114",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_19_1"
},
{
"DOI": "10.1016/j.comppsych.2013.08.012",
"article-title": "Reliability and validity of a Chinese version of the HADS for screening depression and anxiety in psycho‐cardiological outpatients",
"author": "Yang Y",
"doi-asserted-by": "crossref",
"first-page": "215",
"issue": "1",
"journal-title": "Compr Psychiatry",
"key": "e_1_2_13_20_1",
"volume": "55",
"year": "2014"
},
{
"author": "Boland R",
"first-page": "1974",
"key": "e_1_2_13_21_1",
"volume-title": "MD Selective Serotonin Reuptake.Inhibitors",
"year": "2022"
},
{
"DOI": "10.18071/isz.74.0389",
"article-title": "Fluoxetine use is associated with improved survival of patients with COVID‐19 pneumonia: a retrospective case‐control study",
"author": "Németh ZK",
"doi-asserted-by": "crossref",
"first-page": "389",
"issue": "11",
"journal-title": "Ideggyogy Sz",
"key": "e_1_2_13_22_1",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1016/j.pharep.2017.03.011",
"article-title": "Anti‐inflammatory and ameliorative effects of gallic acid on fluoxetine‐induced oxidative stress and liver damage in rats",
"author": "Karimi‐Khouzani O",
"doi-asserted-by": "crossref",
"first-page": "830",
"issue": "4",
"journal-title": "Pharmacol Rep",
"key": "e_1_2_13_23_1",
"volume": "69",
"year": "2017"
},
{
"DOI": "10.1002/prp1002.1231",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_24_1"
},
{
"DOI": "10.1038/nm.3214",
"article-title": "Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs",
"author": "Gulbins E",
"doi-asserted-by": "crossref",
"first-page": "934",
"issue": "7",
"journal-title": "Nat Med",
"key": "e_1_2_13_25_1",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.1016/s2214‐1109x(1021)00448‐00444",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_26_1"
},
{
"DOI": "10.1001/jama.2020.22760",
"article-title": "Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID‐19: a randomized clinical trial",
"author": "Lenze EJ",
"doi-asserted-by": "crossref",
"first-page": "2292",
"issue": "22",
"journal-title": "JAMA",
"key": "e_1_2_13_27_1",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.33090",
"doi-asserted-by": "publisher",
"key": "e_1_2_13_28_1"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/npr2.12327"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Psychiatry and Mental health",
"Pharmacology",
"Clinical Psychology"
],
"subtitle": [],
"title": "Efficacy and safety of adding fluoxetine to the treatment regimen of hospitalized patients with non‐critical\n <scp>COVID</scp>\n ‐19 pneumonia: A double‐blind randomized, placebo‐controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
