Outpatient medications associated with protection from COVID-19 hospitalization
et al., PLOS ONE, doi:10.1371/journal.pone.0282961, Mar 2023
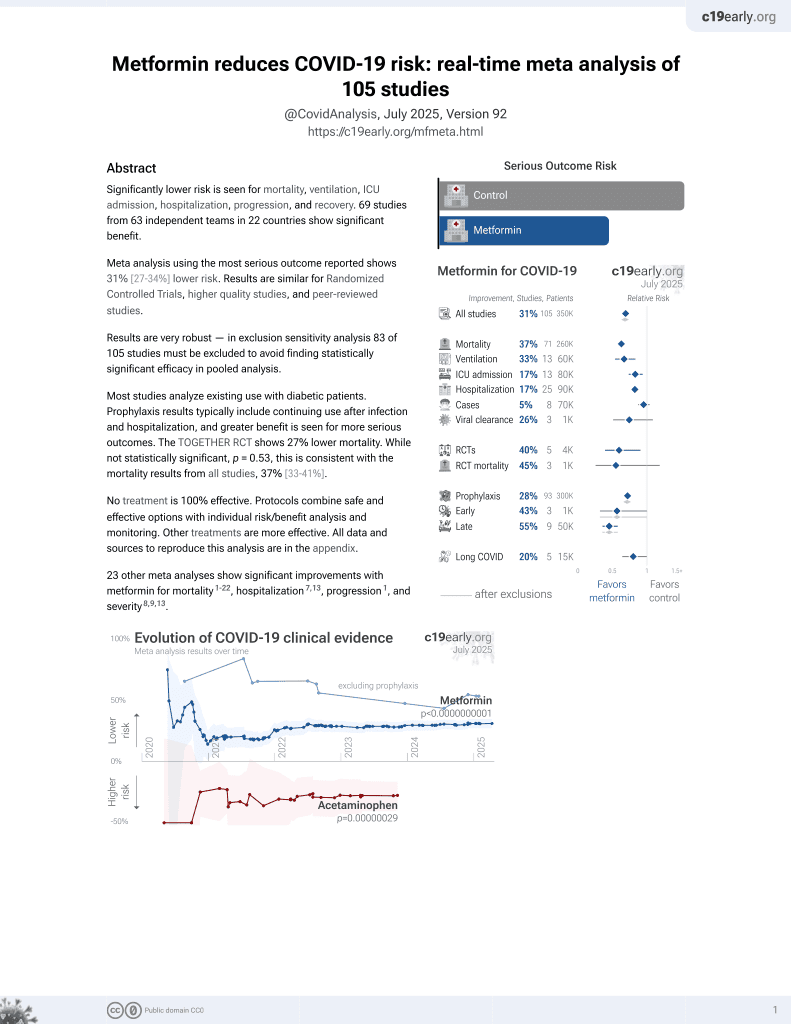
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 3,974,272 COVID-19 patients in the USA, showing 3% lower risk of hospitalization with pre-existing metformin use.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 2.8% lower, OR 0.97, p = 0.004, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sandhu et al., 31 Mar 2023, retrospective, USA, peer-reviewed, mean age 50.7, 7 authors, study period 1 January, 2020 - 31 December, 2020.
Contact: harpal.sandhu@louisville.edu.
Outpatient medications associated with protection from COVID-19 hospitalization
PLOS ONE, doi:10.1371/journal.pone.0282961
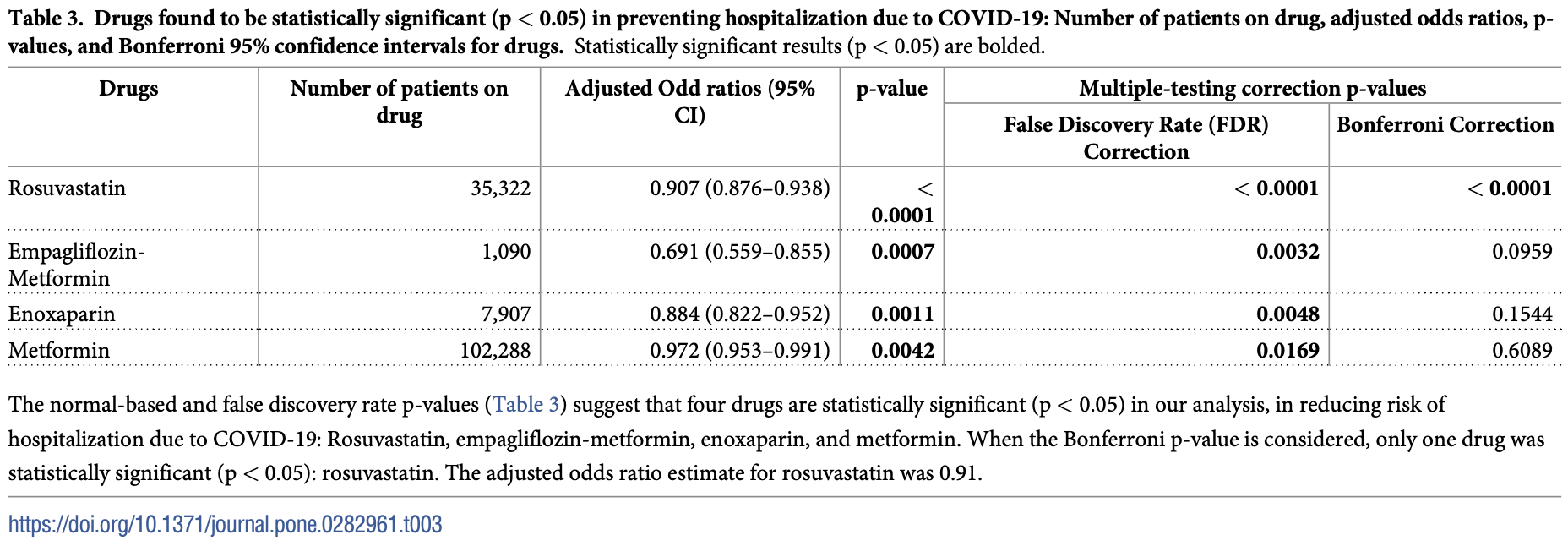
The COVID-19 pandemic remains the pre-eminent global health problem, and yet after more than three years there is still no prophylactic agent against the disease aside from vaccines. The objective of this study was to evaluate whether pre-existing, outpatient medications approved by the US Food and Drug Administration (FDA) reduce the risk of hospitalization due to COVID-19. This was a retrospective cohort study of patients from across the United States infected with COVID-19 in the year 2020. The main outcome was adjusted odds of hospitalization for COVID-19 amongst those positive for the infection. Outcomes were adjusted for known risk factors for severe disease. 3,974,272 patients aged 18 or older with a diagnosis of COVID-19 in 2020 met our inclusion criteria and were included in the analysis. Mean age was 50.7 (SD 18). Of this group, 290,348 patients (7.3%) were hospitalized due to COVID-19, similar to the CDC's reported estimate (7.5%). Four drugs showed protective effects against COVID-19 hospitalization: rosuvastatin (aOR 0.91, p = 0.00000024), empagliflozin-metformin (aOR 0.69, p = 0.003), metformin (aOR 0.97, p = 0.017), and enoxaparin (aOR 0.88, p = 0.0048). Several pre-existing medications for outpatient use may reduce severity of disease and protect against COVID-19 hospitalization. Well-designed clinical trials are needed to assess the efficacy of these agents in a therapeutic or prophylactic setting.
Supporting information S1 Appendix. A full list of all drugs evaluated in this study is included. (DOCX)
Author Contributions
References
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19, N Eng J Med, doi:10.1056/NEJMoa2015432
Arshad, Pertinez, Box, Tatham, Rajioli et al., Prioritisation of anti-SARS-CoV-2 drug repurposing opportunities based on plasma and target site concentrations derived from established human pharmacokinetics, Clin Pharmacol Ther
Baron, Devaux, Colson, Raoult, Rolain, Teicoplanin: an alternative drug for the treatment of COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105944
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bikdeli, Madhavan, Jimenez, Chuich, Dreyfus et al., COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up, J Am Coll Cardiol
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced host response to SARS-CoV-2 drives development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Bocan, Pleiotropic effects of HMG-CoA reductase inhibitors, Curr Opin Investig Drugs
Borba, Val, Sampaio, Alexandre, Melo et al., Effect of high vs low doses of chloroquine diphosophate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial, JAMA Netw Open
Bouillon, Baricault, Semenzato, Botton, Bertrand et al., Association of statins for primary prevention of cardiovascular diseases with hospitalization for COVID-19: A nationwide matched population-based cohort study, J Am Heart Assoc, doi:10.1161/JAHA.121.023357
Bramante, Huling, Tignanelli, Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19, N ENgl J Med, doi:10.1056/NEJMoa2201662
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Cava, Bertoli, Castiglioni, In silico discovery of candidate drugs against Covid-19, Viruses, doi:10.3390/v12040404
Choy, Wong, Kaewpreedee, Sia, Chen et al., Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replications in vitro, Antiviral Res
Coleman, Sisk, Mingo, Nelson, White et al., Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East Respiratory Syndrome coronavirus fusion, J Virol
Dediego, Nieto-Torres, Regla-Nava, Jimenez-Guardeño, Fernandez-Delgado et al., Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirusinfected mice increases survival, J Virol
Dyall, Coleman, Hart, Venkataraman, Holbrook et al., Repurposing of clinically developed drugs for treatment of middle east respiratory syndrome coronavirus infection, Antimicrob Agents Chemother
Fan, Zhang, Liu, Yang, Zheng et al., Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients, Clin Infect Dis
Felsenstein, Herbert, Mcnamara, Hedrich, COVID-19: Immunology and treatment options, Clin Immunol, doi:10.1016/j.clim.2020.108448
Feng, Ni, Little, Xu, Tang, Metformin, macrophage dysfunction and atherosclerosis, Front Immunol, doi:10.3389/fimmu.2021.682853
Ghati, Bhatnagar, Mahendran, Thakur, Prasad et al., Statin and aspirin as adjuvant therapy in hospitalized patients with SARS-CoV-2 infection: a randomized clinical trial (RESIST trial), BMC Infect Dis
Gomeni, Xu, Gao, Bressolle-Gomeni, Model based approach for estimating the dosage regimen of indomethacin, a potential antiviral treatment of patients infected with SARS CoV-2. J Pharmacokinet Pharmacodyn, doi:10.1007/s10928-020-09690-4
Gordon, Jang, Bouhaddou, Xu, Obernier et al., A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Hirano, Murakami, COVID-19: A new virus, but a familiar receptor and cytokine release syndrome, Immunity, doi:10.1016/j.immuni.2020.04.003
Ibrahim, Lowe, Bramante, Shah, Klatt et al., Metformin and COVID-19: focused review of mechanisms and current literature suggesting benefit, Front Endocrinol, doi:10.3389/fendo.2021.587801
Investigators, Atorvastatin versus placebo in patients with covid-19 in intensive care: randomized controlled trial, BMJ, doi:10.1136/bmj-2021-068407
Islam, Parves, Paul, Uddin, Rahman et al., A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2, J Biomed Struct Dyn, doi:10.1080/07391102.2020.1761883
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Kandeel, Al-Nazawi, Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease, Life Sci, doi:10.1016/j.lfs.2020.117627
Kang, Seong, Choi, Kim, Choe et al., In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations by usual doses, Korean J Intern Med
Ke, Peng, Yeh, Artificial intelligence approach fighting COVID-19 with repurposing drugs, Biomed J, doi:10.1016/j.bj.2020.05.001
Klok, Kruip, Van Der Meer, Incidence of thrombotic complications in critically ill ICU patients with COVID-19, Thromb Res, doi:10.1016/j.thromres.2020.04.013
Kollias, Kyriakoulis, Dimakakos, Poulakou, Stergiou et al., Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action, Br J Haematol, doi:10.1111/bjh.16727
Kumar, Saurabh, Narasimha, Maharshi, Efficacy of interferon-b in moderate-to-severe hospitalized cases of COVID-19: a systematic review and meta-analysis, Clin Drug Investig
Lambert, Sandhu, Keen, Xavier, Brokman et al., A strategy to identify event specific hospitalizations in large health claims databases, BMC Health Serv Res, doi:10.1186/s12913-022-08107-x
Lax, Skok, Zechner, Kessler, Kaufmann et al., Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series, Ann Intern Med, doi:10.7326/M20-2566
Liu, Li, Liu, Sun, Chen et al., Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19, Acta Pharm Sin B
Liu, Sumeng, Liu, Liang, Wang et al., Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients, EBioMedicine, doi:10.1016/j.ebiom.2020.102763
Lopes, De, Silva, Furtado, Macedo et al., Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial, Lancet, doi:10.1016/S0140-6736%2821%2901203-4
Mbara, Mato, Driver, Nzuza, Mkhombo et al., Metformin turns 62 in pharmacotherapy: emergency of non-glycaemic effects and potential novel therapeutic applications, Eur J Pharmacol
Middleton, He, Denorme, Campbell, Ng et al., Neutrophil extracellular traps contribute to immunothrombosis in COVID-10 acute respiratory distress syndrome, Blood
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Nilsson-Payant, Uhl, Grimont, Doane, Cohen et al., The NF-kappaB transcriptional footprint is essential for SARS-CoV-2 replication, J Virol
Reiner, Hatamipour, Banach, Pirro, Al-Rasadi et al., Statins and the COVID-19 main protease: in silico evidence on direct interaction, Arch Med Sci, doi:10.5114/aoms.2020.94655
Sadeghipour, Talasz, Rashidi, Sharif-Kashani, Beigmohammadi et al., Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial, J Am Med Assoc, doi:10.1001/jama.2021.4152
Sarma, Sekhar, Prajapat, In-silico homology assisted identification of inhibitor or RNA binding against 2019-nCoV N-protein (N terminal domain), J Biomol Struct Dyn
Shah, Modi, Sagar, Avti, Kaur et al., In silico studies on therapeutic agents for COVID-19: drug repurposing approach, Life Sci, doi:10.1016/j.lfs.2020.117652
Sheahan, Sims, Graham, Menachery, Gralinski et al., Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses, Sci Transl Med, doi:10.1126/scitranslmed.aal3653
Siddiqi, Libby, Ridker, COVID-19 -a vascular disease, Trends Cardiovasc Med, doi:10.1016/j.tcm.2020.10.005
Stebbing, Krishnan, De Bono, Ottaviani, Casalini et al., Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients, EMBO Mol Med, doi:10.15252/emmm.202012697
Stebbing, Phelan, Griffin, Tucker, Oechsle et al., COVID-19: combining antiviral and anti-inflammatory treatments, Lancet Infect Dis, doi:10.1016/S1473-3099%2820%2930132-8
Sunjaya, Sunjaya, Targeting ageing and preventing organ degeneration with metformin, Diabetes Metab, doi:10.1016/j.diabet.2020.09.009
Talasaz, Sadeghipour, Aghakouchakzadeh, Dreyfus, Kakvand et al., Investigating lipid-modulating agents for prevention or treatment of COVID-19: JACC state-of-the-art review, J Am Coll Cardiol, doi:10.1016/j.jacc.2021.08.021
Tang, Bai, Chen, Gong, Li et al., Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy, J Thromb Haemost, doi:10.1111/jth.14817
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomized controlled trial, BMJ
Ton, Gentile, Hseing, Ban, Cherkasov, Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds, Mol Inform
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effective inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial, Lancet
Wichmann, Sperhake, Lutgehetmann, Steurer, Edler et al., Autopsy findings and venous thromboembolism in patients with COVID-19, Ann Intern Med, doi:10.7326/L20-1205
Wu, Jan, Ma, Kuo, Juan et al., Small molecules targeting severe acute respiratory syndrome human coronavirus, Proc Natl Acad Sci, doi:10.1073/pnas.0403596101
Yuan, Chan, Chik, Chan, Tsang et al., Discovery of the FDA-approved drugs bexarotene, cetillistat, dilodohydroxyquinolone, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system, Pharmacol Res
Zhang, Zhao, Zhang, Wang, Li et al., The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): perspective of clinical immunologists from China, Clin Immunol
Zhou, Hou, Shen, Huang, Martin et al., Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2, Cell Discov, doi:10.1038/s41421-020-0153-3
DOI record:
{
"DOI": "10.1371/journal.pone.0282961",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0282961",
"abstract": "<jats:p>The COVID-19 pandemic remains the pre-eminent global health problem, and yet after more than three years there is still no prophylactic agent against the disease aside from vaccines. The objective of this study was to evaluate whether pre-existing, outpatient medications approved by the US Food and Drug Administration (FDA) reduce the risk of hospitalization due to COVID-19. This was a retrospective cohort study of patients from across the United States infected with COVID-19 in the year 2020. The main outcome was adjusted odds of hospitalization for COVID-19 amongst those positive for the infection. Outcomes were adjusted for known risk factors for severe disease. 3,974,272 patients aged 18 or older with a diagnosis of COVID-19 in 2020 met our inclusion criteria and were included in the analysis. Mean age was 50.7 (SD 18). Of this group, 290,348 patients (7.3%) were hospitalized due to COVID-19, similar to the CDC’s reported estimate (7.5%). Four drugs showed protective effects against COVID-19 hospitalization: rosuvastatin (aOR 0.91, p = 0.00000024), empagliflozin-metformin (aOR 0.69, p = 0.003), metformin (aOR 0.97, p = 0.017), and enoxaparin (aOR 0.88, p = 0.0048). Several pre-existing medications for outpatient use may reduce severity of disease and protect against COVID-19 hospitalization. Well-designed clinical trials are needed to assess the efficacy of these agents in a therapeutic or prophylactic setting.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5500-8910",
"affiliation": [],
"authenticated-orcid": true,
"family": "Sandhu",
"given": "Harpal Singh",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lambert",
"given": "Joshua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Steckler",
"given": "Zach",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Lee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stromberg",
"given": "Arnold",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramirez",
"given": "Julio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Chi-fu Jeffrey",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2023,
3,
31
]
],
"date-time": "2023-03-31T17:57:47Z",
"timestamp": 1680285467000
},
"deposited": {
"date-parts": [
[
2023,
3,
31
]
],
"date-time": "2023-03-31T17:58:22Z",
"timestamp": 1680285502000
},
"editor": [
{
"affiliation": [],
"family": "Kannan",
"given": "Lakshmi",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100000092",
"award": [
"R21LM013683-01"
],
"doi-asserted-by": "publisher",
"name": "U.S. National Library of Medicine"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T04:58:11Z",
"timestamp": 1680325091050
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
3,
31
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2023,
3,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
31
]
],
"date-time": "2023-03-31T00:00:00Z",
"timestamp": 1680220800000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0282961",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0282961",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2023,
3,
31
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
31
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1002/minf.202000028",
"article-title": "Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds.",
"author": "A Ton",
"doi-asserted-by": "crossref",
"first-page": "e2000028",
"journal-title": "Mol Inform",
"key": "pone.0282961.ref001",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.15252/emmm.202012697",
"article-title": "Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients",
"author": "J Stebbing",
"doi-asserted-by": "crossref",
"first-page": "e12697",
"journal-title": "EMBO Mol Med",
"key": "pone.0282961.ref002",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.bj.2020.05.001",
"article-title": "Artificial intelligence approach fighting COVID-19 with repurposing drugs.",
"author": "Y Ke",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Biomed J",
"key": "pone.0282961.ref003",
"volume": "43",
"year": "2020"
},
{
"article-title": "A molecular modeling approach to identify effective antiviral phytochemicals against the main protease of SARS-CoV-2.",
"author": "R Islam",
"first-page": "3213",
"journal-title": "J Biomed Struct Dyn",
"key": "pone.0282961.ref004",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1753580",
"article-title": "In-silico homology assisted identification of inhibitor or RNA binding against 2019-nCoV N-protein (N terminal domain).",
"author": "P Sarma",
"doi-asserted-by": "crossref",
"first-page": "2724",
"journal-title": "J Biomol Struct Dyn",
"key": "pone.0282961.ref005",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.3390/v12040404",
"article-title": "In silico discovery of candidate drugs against Covid-19.",
"author": "C Cava",
"doi-asserted-by": "crossref",
"first-page": "404",
"issue": "4",
"journal-title": "Viruses",
"key": "pone.0282961.ref006",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117652",
"article-title": "In silico studies on therapeutic agents for COVID-19: drug repurposing approach",
"author": "B Shah",
"doi-asserted-by": "crossref",
"first-page": "117652",
"journal-title": "Life Sci",
"key": "pone.0282961.ref007",
"volume": "252",
"year": "2020"
},
{
"DOI": "10.5114/aoms.2020.94655",
"article-title": "Statins and the COVID-19 main protease: in silico evidence on direct interaction.",
"author": "Z Reiner",
"doi-asserted-by": "crossref",
"first-page": "490",
"journal-title": "Arch Med Sci.",
"key": "pone.0282961.ref008",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117627",
"article-title": "Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease",
"author": "M Kandeel",
"doi-asserted-by": "crossref",
"first-page": "117627",
"journal-title": "Life Sci",
"key": "pone.0282961.ref009",
"volume": "251",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"article-title": "COVID-19: combining antiviral and anti-inflammatory treatments",
"author": "J Stebbing",
"doi-asserted-by": "crossref",
"first-page": "400",
"journal-title": "Lancet Infect Dis",
"key": "pone.0282961.ref010",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"article-title": "A SARS-CoV-2 protein interaction map reveals targets for drug repurposing",
"author": "DE Gordon",
"doi-asserted-by": "crossref",
"first-page": "459",
"journal-title": "Nature",
"key": "pone.0282961.ref011",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1038/s41421-020-0153-3",
"article-title": "Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2",
"author": "Y Zhou",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Cell Discov",
"key": "pone.0282961.ref012",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1128/AAC.03036-14",
"article-title": "Repurposing of clinically developed drugs for treatment of middle east respiratory syndrome coronavirus infection",
"author": "J Dyall",
"doi-asserted-by": "crossref",
"first-page": "4885",
"journal-title": "Antimicrob Agents Chemother",
"key": "pone.0282961.ref013",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs",
"author": "S Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819",
"journal-title": "Antimicrob Agents Chemother",
"key": "pone.0282961.ref014",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01429-16",
"article-title": "Abelson kinase inhibitors are potent inhibitors of severe acute respiratory syndrome coronavirus and Middle East Respiratory Syndrome coronavirus fusion",
"author": "CM Coleman",
"doi-asserted-by": "crossref",
"first-page": "8924",
"journal-title": "J Virol",
"key": "pone.0282961.ref015",
"volume": "90",
"year": "2020"
},
{
"DOI": "10.1007/s10928-020-09690-4",
"article-title": "Model based approach for estimating the dosage regimen of indomethacin, a potential antiviral treatment of patients infected with SARS CoV-2.",
"author": "R Gomeni",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "J Pharmacokinet Pharmacodyn",
"key": "pone.0282961.ref016",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1002/cpt.1909",
"article-title": "Prioritisation of anti-SARS-CoV-2 drug repurposing opportunities based on plasma and target site concentrations derived from established human pharmacokinetics",
"author": "U Arshad",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "Clin Pharmacol Ther",
"key": "pone.0282961.ref017",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa623",
"article-title": "Connecting hydroxychloroquine in vitro antiviral activity to in vivo concentration for prediction of antiviral effect: a critical step in treating COVID-19 patients",
"author": "J Fan",
"doi-asserted-by": "crossref",
"first-page": "3232",
"journal-title": "Clin Infect Dis",
"key": "pone.0282961.ref018",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105944",
"article-title": "Teicoplanin: an alternative drug for the treatment of COVID-19.",
"author": "SA Baron",
"doi-asserted-by": "crossref",
"first-page": "105944",
"journal-title": "Int J Antimicrob Agents",
"key": "pone.0282961.ref019",
"volume": "55",
"year": "2020"
},
{
"article-title": "Discovery of the FDA-approved drugs bexarotene, cetillistat, dilodohydroxyquinolone, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system Pharmacol Res.",
"author": "S Yuan",
"first-page": "104960",
"key": "pone.0282961.ref020",
"volume": "159",
"year": "2020"
},
{
"article-title": "Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19 Acta Pharm Sin B.",
"author": "X Liu",
"first-page": "1205",
"key": "pone.0282961.ref021",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"article-title": "Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replications in vitro",
"author": "K Choy",
"doi-asserted-by": "crossref",
"first-page": "104786",
"journal-title": "Antiviral Res",
"key": "pone.0282961.ref022",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.3904/kjim.2020.157",
"article-title": "In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations by usual doses",
"author": "CK Kang",
"doi-asserted-by": "crossref",
"first-page": "782",
"journal-title": "Korean J Intern Med",
"key": "pone.0282961.ref023",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1073/pnas.0403596101",
"article-title": "Small molecules targeting severe acute respiratory syndrome human coronavirus",
"author": "CY Wu",
"doi-asserted-by": "crossref",
"first-page": "10012",
"journal-title": "Proc Natl Acad Sci USA",
"key": "pone.0282961.ref024",
"volume": "101",
"year": "2004"
},
{
"DOI": "10.1126/scitranslmed.aal3653",
"article-title": "Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses.",
"author": "TP Sheahan",
"doi-asserted-by": "crossref",
"first-page": "eaal3656",
"journal-title": "Sci Transl Med.",
"key": "pone.0282961.ref025",
"volume": "9",
"year": "2017"
},
{
"article-title": "Remdesivir and chloroquine effective inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro.",
"author": "M Wang",
"first-page": "2690271",
"journal-title": "Cell Res",
"key": "pone.0282961.ref026",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.8857",
"article-title": "Effect of high vs low doses of chloroquine diphosophate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial.",
"author": "MGS Borba",
"doi-asserted-by": "crossref",
"first-page": "e208857",
"journal-title": "JAMA Netw Open",
"key": "pone.0282961.ref027",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1849",
"article-title": "Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomized controlled trial",
"author": "W Tang",
"doi-asserted-by": "crossref",
"first-page": "m1849",
"journal-title": "BMJ",
"key": "pone.0282961.ref028",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicentre trial",
"author": "Y Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "pone.0282961.ref029",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19",
"author": "B Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "pone.0282961.ref030",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.immuni.2020.04.003",
"article-title": "COVID-19: A new virus, but a familiar receptor and cytokine release syndrome",
"author": "T Hirano",
"doi-asserted-by": "crossref",
"first-page": "731",
"journal-title": "Immunity",
"key": "pone.0282961.ref031",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1007/s40261-021-01092-9",
"article-title": "Efficacy of interferon-b in moderate-to-severe hospitalized cases of COVID-19: a systematic review and meta-analysis.",
"author": "S Kumar",
"doi-asserted-by": "crossref",
"first-page": "1037",
"journal-title": "Clin Drug Investig",
"key": "pone.0282961.ref032",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19 –final report",
"author": "JH Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "pone.0282961.ref033",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108448",
"article-title": "COVID-19: Immunology and treatment options",
"author": "S Felsenstein",
"doi-asserted-by": "crossref",
"first-page": "108448",
"journal-title": "Clin Immunol",
"key": "pone.0282961.ref034",
"volume": "215",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108393",
"article-title": "The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): perspective of clinical immunologists from China.",
"author": "W Zhang",
"doi-asserted-by": "crossref",
"first-page": "108393",
"journal-title": "Clin Immunol",
"key": "pone.0282961.ref035",
"volume": "214",
"year": "2020"
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19",
"author": "JB Moore",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Science",
"key": "pone.0282961.ref036",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"article-title": "Imbalanced host response to SARS-CoV-2 drives development of COVID-19",
"author": "D Blanco-Melo",
"doi-asserted-by": "crossref",
"first-page": "1036",
"journal-title": "Cell",
"key": "pone.0282961.ref037",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2020.102763",
"article-title": "Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients.",
"author": "J Liu",
"doi-asserted-by": "crossref",
"first-page": "102763",
"journal-title": "EBioMedicine",
"key": "pone.0282961.ref038",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19.",
"author": "M Ackermann",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "N Eng J Med.",
"key": "pone.0282961.ref039",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.7326/L20-1205",
"article-title": "Autopsy findings and venous thromboembolism in patients with COVID-19",
"author": "D Wichmann",
"doi-asserted-by": "crossref",
"first-page": "1029",
"journal-title": "Ann Intern Med",
"key": "pone.0282961.ref040",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.7326/M20-2566",
"article-title": "Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series",
"author": "SF Lax",
"doi-asserted-by": "crossref",
"first-page": "350",
"journal-title": "Ann Intern Med",
"key": "pone.0282961.ref041",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1016/j.jacc.2020.04.031",
"article-title": "COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up",
"author": "B Bikdeli",
"doi-asserted-by": "crossref",
"first-page": "2950",
"journal-title": "J Am Coll Cardiol",
"key": "pone.0282961.ref042",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1111/jth.14817",
"article-title": "Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy",
"author": "N Tang",
"doi-asserted-by": "crossref",
"first-page": "1094",
"journal-title": "J Thromb Haemost",
"key": "pone.0282961.ref043",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1111/bjh.16727",
"article-title": "Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action",
"author": "A Kollias",
"doi-asserted-by": "crossref",
"first-page": "846",
"journal-title": "Br J Haematol",
"key": "pone.0282961.ref044",
"volume": "189",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.04.013",
"article-title": "Incidence of thrombotic complications in critically ill ICU patients with COVID-19",
"author": "FA Klok",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "Thromb Res",
"key": "pone.0282961.ref045",
"volume": "191",
"year": "2020"
},
{
"key": "pone.0282961.ref046",
"unstructured": "COVID-19 Research Database. https://covid19researchdatabase.org"
},
{
"DOI": "10.1186/s12913-022-08107-x",
"article-title": "A strategy to identify event specific hospitalizations in large health claims databases.",
"author": "J Lambert",
"doi-asserted-by": "crossref",
"first-page": "705",
"journal-title": "BMC Health Serv Res",
"key": "pone.0282961.ref047",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.tcm.2020.10.005",
"article-title": "COVID-19 –a vascular disease.",
"author": "HK Siddiqi",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Trends Cardiovasc Med",
"key": "pone.0282961.ref048",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1161/JAHA.121.023357",
"article-title": "Association of statins for primary prevention of cardiovascular diseases with hospitalization for COVID-19: A nationwide matched population-based cohort study",
"author": "K Bouillon",
"doi-asserted-by": "crossref",
"first-page": "e023357",
"journal-title": "J Am Heart Assoc",
"key": "pone.0282961.ref049",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.jacc.2021.08.021",
"article-title": "Investigating lipid-modulating agents for prevention or treatment of COVID-19: JACC state-of-the-art review",
"author": "AH Talasaz",
"doi-asserted-by": "crossref",
"first-page": "1635",
"journal-title": "J Am Coll Cardiol",
"key": "pone.0282961.ref050",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.1186/s12879-022-07570-5",
"article-title": "Statin and aspirin as adjuvant therapy in hospitalized patients with SARS-CoV-2 infection: a randomized clinical trial (RESIST trial).",
"author": "N Ghati",
"doi-asserted-by": "crossref",
"first-page": "606",
"journal-title": "BMC Infect Dis",
"key": "pone.0282961.ref051",
"volume": "22",
"year": "2022"
},
{
"article-title": "Atorvastatin versus placebo in patients with covid-19 in intensive care: randomized controlled trial.",
"author": "INSPIRATION-S investigators",
"first-page": "e068407",
"journal-title": "BMJ",
"key": "pone.0282961.ref052",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1182/blood.2020007008",
"article-title": "Neutrophil extracellular traps contribute to immunothrombosis in COVID-10 acute respiratory distress syndrome",
"author": "EA Middleton",
"doi-asserted-by": "crossref",
"first-page": "1169",
"journal-title": "Blood",
"key": "pone.0282961.ref053",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01257-21",
"article-title": "The NF-kappaB transcriptional footprint is essential for SARS-CoV-2 replication",
"author": "BE Nilsson-Payant",
"doi-asserted-by": "crossref",
"first-page": "e0125721",
"journal-title": "J Virol",
"key": "pone.0282961.ref054",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1128/JVI.02576-13",
"article-title": "Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival",
"author": "ML DeDiego",
"doi-asserted-by": "crossref",
"first-page": "913",
"journal-title": "J Virol",
"key": "pone.0282961.ref055",
"volume": "88",
"year": "2014"
},
{
"article-title": "Pleiotropic effects of HMG-CoA reductase inhibitors.",
"author": "TMA Bocan",
"first-page": "1312",
"journal-title": "Curr Opin Investig Drugs",
"key": "pone.0282961.ref056",
"volume": "3",
"year": "2002"
},
{
"DOI": "10.1001/jama.2021.4152",
"article-title": "Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial",
"author": "P Sadeghipour",
"doi-asserted-by": "crossref",
"first-page": "1620",
"journal-title": "J Am Med Assoc",
"key": "pone.0282961.ref057",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01203-4",
"article-title": "Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial.",
"author": "RD Lopes",
"doi-asserted-by": "crossref",
"first-page": "2253",
"journal-title": "Lancet",
"key": "pone.0282961.ref058",
"volume": "397",
"year": "2021"
},
{
"key": "pone.0282961.ref059",
"unstructured": "https://clinicaltrials.gov/ct2/show/NCT04472611?term=colchicine+rosuvastatin&cond=COVID-19&draw=2&rank=1. Accessed December 29, 2022."
},
{
"key": "pone.0282961.ref060",
"unstructured": "American Society of Hematology Guidelines on Use of Anticoagulation in Patients with COVID-19. www.hematology. Org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines/ash-guidelines-on-use-of-anticoagulation-in-pateints-with-covid-19. Accessed September 5, 2022."
},
{
"DOI": "10.1016/j.diabet.2020.09.009",
"article-title": "Targeting ageing and preventing organ degeneration with metformin",
"author": "AP Sunjaya",
"doi-asserted-by": "crossref",
"first-page": "101203",
"journal-title": "Diabetes Metab",
"key": "pone.0282961.ref061",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.682853",
"article-title": "Metformin, macrophage dysfunction and atherosclerosis",
"author": "X Feng",
"doi-asserted-by": "crossref",
"first-page": "682853",
"journal-title": "Front Immunol",
"key": "pone.0282961.ref062",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2021.173934",
"article-title": "Metformin turns 62 in pharmacotherapy: emergency of non-glycaemic effects and potential novel therapeutic applications",
"author": "KC Mbara",
"doi-asserted-by": "crossref",
"first-page": "173934",
"journal-title": "Eur J Pharmacol",
"key": "pone.0282961.ref063",
"volume": "898",
"year": "2021"
},
{
"DOI": "10.3389/fendo.2021.587801",
"article-title": "Metformin and COVID-19: focused review of mechanisms and current literature suggesting benefit.",
"author": "S Ibrahim",
"doi-asserted-by": "crossref",
"first-page": "587801",
"journal-title": "Front Endocrinol (Lausanne)",
"key": "pone.0282961.ref064",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2201662",
"article-title": "Randomized trial of metformin, ivermectin, and fluvoxamine for Covid-19",
"author": "CT Bramante",
"doi-asserted-by": "crossref",
"first-page": "599",
"journal-title": "N ENgl J Med",
"key": "pone.0282961.ref065",
"volume": "387",
"year": "2022"
},
{
"key": "pone.0282961.ref066",
"unstructured": "https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html"
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0282961"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Outpatient medications associated with protection from COVID-19 hospitalization",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "18"
}