Nitazoxanide in Patients Hospitalized With COVID-19 Pneumonia: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.844728, NCT04561219, Apr 2022
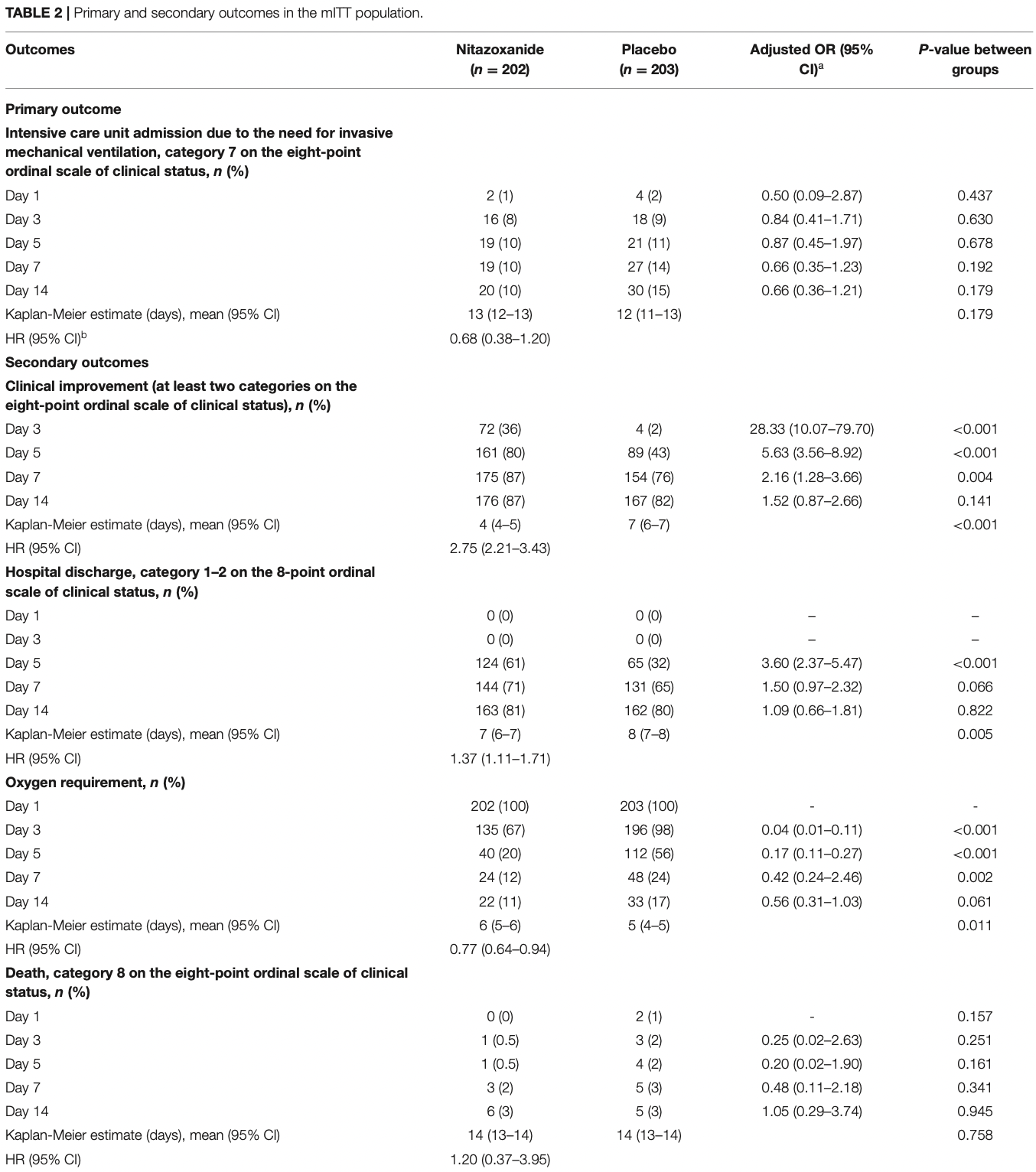
RCT late stage patients with COVID-19 pneumonia, 202 treated with nitazoxanide and 203 placebo patients, showing improved recovery, but no significant difference in mortality.
|
risk of death, 4.9% higher, RR 1.05, p = 0.94, treatment 6 of 202 (3.0%), control 5 of 203 (2.5%), adjusted per study, odds ratio converted to relative risk, multivariable, day 14.
|
|
risk of ICU admission, 30.5% lower, RR 0.69, p = 0.18, treatment 20 of 202 (9.9%), control 30 of 203 (14.8%), NNT 21, adjusted per study, odds ratio converted to relative risk, multivariable, day 14.
|
|
risk of oxygen therapy, 39.7% lower, RR 0.60, p = 0.06, treatment 22 of 202 (10.9%), control 33 of 203 (16.3%), NNT 19, adjusted per study, odds ratio converted to relative risk, multivariable, day 14.
|
|
time to improvement, 63.6% lower, HR 0.36, p < 0.001, treatment 202, control 203, inverted to make HR<1 favor treatment, Kaplan-Meier.
|
|
improvement, 34.2% better, OR 0.66, p = 0.14, treatment 202, control 203, adjusted per study, inverted to make OR<1 favor treatment, multivariable, day 14, RR approximated with OR.
|
|
time to discharge, 27.0% lower, HR 0.73, p = 0.004, treatment 202, control 203, inverted to make HR<1 favor treatment, Kaplan-Meier.
|
|
discharge, 8.3% lower, OR 0.92, p = 0.82, treatment 202, control 203, adjusted per study, inverted to make OR<1 favor treatment, multivariable, day 14, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Rocco et al., 13 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, median age 56.0, 37 authors, study period 20 April, 2020 - 2 October, 2020, trial NCT04561219 (history).
Contact: prmrocco@biof.ufrj.br.
Nitazoxanide in Patients Hospitalized With COVID-19 Pneumonia: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial
Frontiers in Medicine, doi:10.3389/fmed.2022.844728
Background: Nitazoxanide exerts antiviral activity in vitro and in vivo and anti-inflammatory effects, but its impact on patients hospitalized with COVID-19 pneumonia is uncertain. Methods: A multicentre, randomized, double-blind, placebo-controlled trial was conducted in 19 hospitals in Brazil. Hospitalized adult patients requiring supplemental oxygen, with COVID-19 symptoms and a chest computed tomography scan suggestive of viral pneumonia or positive RT-PCR test for COVID-19 were enrolled. Patients were randomized 1:1 to receive nitazoxanide (500 mg) or placebo, 3 times daily, for 5 days, and were followed for 14 days. The primary outcome was intensive care unit admission due to the need for invasive mechanical ventilation. Secondary outcomes included clinical improvement, hospital discharge, oxygen requirements, death, and adverse events within 14 days. Rocco et al.
Nitazoxanide in COVID-19 Pneumonia Patients Results: Of the 498 patients, 405 (202 in the nitazoxanide group and 203 in the placebo group) were included in the analyses. Admission to the intensive care unit did not differ between the groups (hazard ratio [95% confidence interval], 0.68 [0.38-1.20], p = 0.179); death rates also did not differ. Nitazoxanide improved the clinical outcome (2. 75 [2.21-3.43], p < 0.0001), time to hospital discharge (1.37 [1.11-1.71], p = 0.005), and reduced oxygen requirements (0.77 [0.64-0.94], p = 0.011). C-reactive protein, D-dimer, and ferritin levels were lower in the nitazoxanide group than the placebo group on day 7. No serious adverse events were observed. Conclusions: Nitazoxanide, compared with placebo, did not prevent admission to the intensive care unit for patients hospitalized with COVID-19 pneumonia.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by Brazilian National Commission for Research Ethics (CAAE:30662420.0.1001.0008). The patients/participants provided their written informed consent to participate in this study.
AUTHOR CONTRIBUTIONS PR, PS, and FC were involved in the conception and design of the study. PT, ER, JJ, FH, RÁ, JS, MM, KB, LB, AC, AJ, JA-F, LC, PA, AM, RC, MO, RS, and CS were responsible for recruitment and clinical care of the patients. AF was responsible for laboratory analyses. KF, PP, and JL-e-S contributed to data interpretation and critical review of the manuscript. RL, SM, CS, and CM were responsible for the statistical analysis. NF, PM-S, CN, and DC contributed to data collection. All authors reviewed and approved the final version of the manuscript. Authority for Studies and Projects (FINEP), Brasília, Brazil (number: 01.20.0003.00).
SUPPLEMENTARY MATERIAL The The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher...
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19 -preliminary report, N Engl J Med, doi:10.1056/NEJMoa200776
Berlin, Gulick, Martinez, Severe Covid-19
Blum, Cimerman, Hunter, Tierno, Lacerda et al., Nitazoxanide superiority to placebo to treat moderate COVID-19 -a pilot prove of concept randomized double-blind clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100981
Core, R: A Language and Environment for Statistical Computing
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of Coronavirus Disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Haffizulla, Hartman, Hoppers, Resnick, Samudrala et al., Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(14)70717-0
Harris, Taylor, Minor, Elliott, Fernandez et al., The REDCap consortium: building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Hong, Kim, Song, Choi, Lee et al., Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice, Int Immunopharmacol, doi:10.1016/j.intimp.2012.03.002
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus Remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Munch, Myatra, Vijayaraghavan, Saseedharan, Benfield et al., Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial, JAMA, doi:10.1001/jama.2021.18295
Raut, Huy, Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave?, Lancet Respir Med, doi:10.1016/S2213-2600(21)00265-4
Robba, Battaglini, Pelosi, Rocco, Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2, Exp Rev Respir Med, doi:10.1080/17476348.2020.1778470
Rocco, Silva, Cruz, Junior, Tierno et al., Early use of nitazoxanide in mild Covid-19 disease: randomised, placebocontrolled trial, Eur Respir J, doi:10.1183/13993003.03725-2020
Rossignol, Bardin, Fulgencio, Mogelnicki, Bréchot, A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101310
Schulz, Altman, Moher, Consort, statement: updated guidelines for reporting parallel group randomised trials, BMJ, doi:10.1136/bmj.c332
Shou, Kong, Wang, Tang, Wang et al., Tizoxanide inhibits inflammation in LPS-activated RAW264.7 macrophages via the suppression of NF-kappaB and MAPK activation, Inflammation, doi:10.1007/s10753-019-00994-3
Singh, Singh, Joshi, Misra, Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India, Diabetes Metab Syndr, doi:10.1016/j.dsx.2021.05.019
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
DOI record:
{
"DOI": "10.3389/fmed.2022.844728",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2022.844728",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Nitazoxanide exerts antiviral activity <jats:italic>in vitro</jats:italic> and <jats:italic>in vivo</jats:italic> and anti-inflammatory effects, but its impact on patients hospitalized with COVID-19 pneumonia is uncertain.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A multicentre, randomized, double-blind, placebo-controlled trial was conducted in 19 hospitals in Brazil. Hospitalized adult patients requiring supplemental oxygen, with COVID-19 symptoms and a chest computed tomography scan suggestive of viral pneumonia or positive RT-PCR test for COVID-19 were enrolled. Patients were randomized 1:1 to receive nitazoxanide (500 mg) or placebo, 3 times daily, for 5 days, and were followed for 14 days. The primary outcome was intensive care unit admission due to the need for invasive mechanical ventilation. Secondary outcomes included clinical improvement, hospital discharge, oxygen requirements, death, and adverse events within 14 days.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of the 498 patients, 405 (202 in the nitazoxanide group and 203 in the placebo group) were included in the analyses. Admission to the intensive care unit did not differ between the groups (hazard ratio [95% confidence interval], 0.68 [0.38–1.20], <jats:italic>p</jats:italic> = 0.179); death rates also did not differ. Nitazoxanide improved the clinical outcome (2.75 [2.21–3.43], <jats:italic>p</jats:italic> &lt; 0.0001), time to hospital discharge (1.37 [1.11–1.71], <jats:italic>p</jats:italic> = 0.005), and reduced oxygen requirements (0.77 [0.64–0.94], <jats:italic>p</jats:italic> = 0.011). C-reactive protein, D-dimer, and ferritin levels were lower in the nitazoxanide group than the placebo group on day 7. No serious adverse events were observed.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Nitazoxanide, compared with placebo, did not prevent admission to the intensive care unit for patients hospitalized with COVID-19 pneumonia.</jats:p></jats:sec><jats:sec><jats:title>Clinical Trial Registration</jats:title><jats:p>Brazilian Registry of Clinical Trials (REBEC) RBR88bs9x; <jats:ext-link>ClinicalTrials.gov</jats:ext-link>, NCT04561219.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2022.844728"
],
"author": [
{
"affiliation": [],
"family": "Rocco",
"given": "Patricia R. M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Silva",
"given": "Pedro L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cruz",
"given": "Fernanda F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tierno",
"given": "Paulo F. G. M. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rabello",
"given": "Eucir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Junior",
"given": "Jéfiton Cordeiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haag",
"given": "Firmino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Ávila",
"given": "Renata E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Silva",
"given": "Joana D. G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mamede",
"given": "Mariana M. S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buchele",
"given": "Konrad S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barbosa",
"given": "Luiz C. V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabral",
"given": "Anna C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Junqueira",
"given": "Antônio A. F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Araújo-Filho",
"given": "João A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Costa",
"given": "Lucianna A. T. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alvarenga",
"given": "Pedro P. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moura",
"given": "Alexandre S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carajeleascow",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Oliveira",
"given": "Mirella C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Roberta G. F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soares",
"given": "Cynthia R. P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandes",
"given": "Ana Paula S. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fonseca",
"given": "Flavio Guimarães",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camargos",
"given": "Vidyleison Neves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reis",
"given": "Julia de Souza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Franchini",
"given": "Kleber G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luiz",
"given": "Ronir R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morais",
"given": "Sirlei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sverdloff",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martins",
"given": "Camila Marinelli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Felix",
"given": "Nathane S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mattos-Silva",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nogueira",
"given": "Caroline M. B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caldeira",
"given": "Dayene A. F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelosi",
"given": "Paolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lapa-e-Silva",
"given": "José R.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
4,
13
]
],
"date-time": "2022-04-13T05:56:55Z",
"timestamp": 1649829415000
},
"deposited": {
"date-parts": [
[
2022,
4,
13
]
],
"date-time": "2022-04-13T05:56:59Z",
"timestamp": 1649829419000
},
"funder": [
{
"DOI": "10.13039/501100004809",
"doi-asserted-by": "publisher",
"name": "Financiadora de Estudos e Projetos"
},
{
"DOI": "10.13039/501100003593",
"doi-asserted-by": "publisher",
"name": "Conselho Nacional de Desenvolvimento Científico e Tecnológico"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
13
]
],
"date-time": "2022-04-13T06:12:50Z",
"timestamp": 1649830370058
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
4,
13
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
13
]
],
"date-time": "2022-04-13T00:00:00Z",
"timestamp": 1649808000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.844728/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
4,
13
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
13
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1080/17476348.2020.1778470",
"article-title": "Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2",
"author": "Robba",
"doi-asserted-by": "publisher",
"first-page": "865",
"journal-title": "Exp Rev Respir Med.",
"key": "B1",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of Coronavirus Disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N Engl J Med.",
"key": "B2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009575",
"article-title": "Severe Covid-19",
"author": "Berlin",
"doi-asserted-by": "publisher",
"first-page": "2451",
"journal-title": "N Engl J Med.",
"key": "B3",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa200776",
"article-title": "Remdesivir for the treatment of Covid-19 - preliminary report",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N Engl J Med.",
"key": "B4",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "B5",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1569",
"journal-title": "Lancet.",
"key": "B6",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1183/13993003.03725-2020",
"article-title": "Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial",
"author": "Rocco",
"doi-asserted-by": "publisher",
"first-page": "2003725",
"journal-title": "Eur Respir J.",
"key": "B7",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2012.03.002",
"article-title": "Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice",
"author": "Hong",
"doi-asserted-by": "publisher",
"first-page": "23",
"journal-title": "Int Immunopharmacol.",
"key": "B8",
"volume": "13",
"year": "2012"
},
{
"DOI": "10.1007/s10753-019-00994-3",
"article-title": "Tizoxanide inhibits inflammation in LPS-activated RAW264.7 macrophages via the suppression of NF-kappaB and MAPK activation",
"author": "Shou",
"doi-asserted-by": "publisher",
"journal-title": "Inflammation.",
"key": "B9",
"year": "2019"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"article-title": "Nitazoxanide superiority to placebo to treat moderate COVID-19 - a pilot prove of concept randomized double-blind clinical trial",
"author": "Blum",
"doi-asserted-by": "publisher",
"first-page": "100981",
"journal-title": "EClinicalMedicine.",
"key": "B10",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2022.101310",
"article-title": "A randomized double-blind placebo-controlled clinical trial of nitazoxanide for treatment of mild or moderate COVID-19",
"author": "Rossignol",
"doi-asserted-by": "publisher",
"first-page": "101310",
"journal-title": "EClinicalMedicine.",
"key": "B11",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"article-title": "Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial",
"author": "Haffizulla",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis.",
"key": "B12",
"year": "2014"
},
{
"DOI": "10.1136/bmj.c332",
"article-title": "CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials",
"author": "Schulz",
"doi-asserted-by": "publisher",
"first-page": "c332",
"journal-title": "BMJ.",
"key": "B13",
"volume": "340",
"year": "2010"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap consortium: building an international community of software platform partners",
"author": "Harris",
"doi-asserted-by": "publisher",
"first-page": "103208",
"journal-title": "J Biomed Inform.",
"key": "B14",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus Remdesivir for hospitalized adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"journal-title": "N Engl J Med.",
"key": "B15",
"volume": "384",
"year": "2021"
},
{
"key": "B16",
"volume-title": "R: A Language and Environment for Statistical Computing",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N Engl J Med.",
"key": "B17",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.18295",
"article-title": "Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial",
"author": "Munch",
"doi-asserted-by": "publisher",
"first-page": "1807",
"journal-title": "JAMA.",
"key": "B18",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2021.05.019",
"article-title": "Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "102146",
"journal-title": "Diabetes Metab Syndr.",
"key": "B19",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00265-4",
"article-title": "Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave?",
"author": "Raut",
"doi-asserted-by": "publisher",
"first-page": "e77",
"journal-title": "Lancet Respir Med.",
"key": "B20",
"volume": "9",
"year": "2021"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.844728/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Nitazoxanide in Patients Hospitalized With COVID-19 Pneumonia: A Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "9"
}