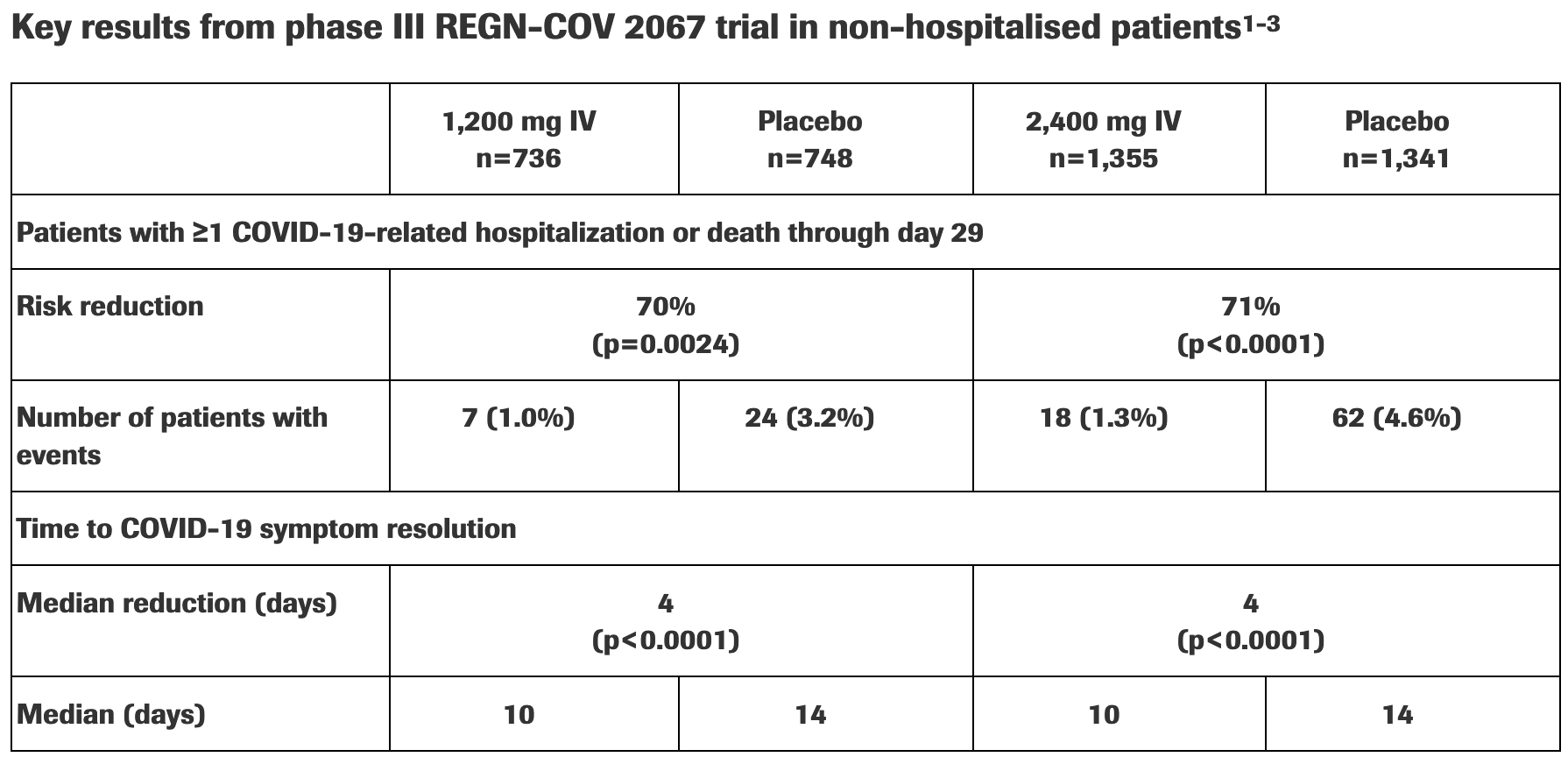
New phase III data shows investigational antibody cocktail casirivimab and imdevimab reduced hospitalisation or death by 70% in non-hospitalised patients with COVID-19
, Press Release, Mar 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Press release for new phase III data showing lower hospitalization/mortality, and faster symptom resolution among the subset of patients with at least one risk factor.
Some variants may escape antibodies1.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants2-8.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments9.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death/hospitalization, 71.3% lower, RR 0.29, p < 0.001, treatment 18 of 1,355 (1.3%), control 62 of 1,341 (4.6%), NNT 30, 2,400mg IV, ≥1 risk factor.
|
|
risk of death/hospitalization, 70.4% lower, RR 0.30, p = 0.003, treatment 7 of 736 (1.0%), control 24 of 748 (3.2%), NNT 44, 1,200mg IV, ≥1 risk factor.
|
|
recovery time, 28.6% lower, relative time 0.71, p < 0.001, treatment 1,355, control 1,341, 2,400mg IV, ≥1 risk factor.
|
|
recovery time, 28.6% lower, relative time 0.71, p < 0.001, treatment 736, control 748, 1,200mg IV, ≥1 risk factor.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
2.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
3.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
4.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
5.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
6.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
7.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Regeneron et al., 23 Mar 2021, Randomized Controlled Trial, USA, preprint, 1 author.
