XAV-19 a Glyco-Humanized polyclonal antibody targeting SARS-CoV-2 accelerates the recovery of mild to moderate COVID-19 patients and keeps its neutralizing activity against Omicron and its subvariants
et al., medRxiv, doi:10.1101/2023.10.09.23296726, EUROXAV, NCT04928430, Oct 2023
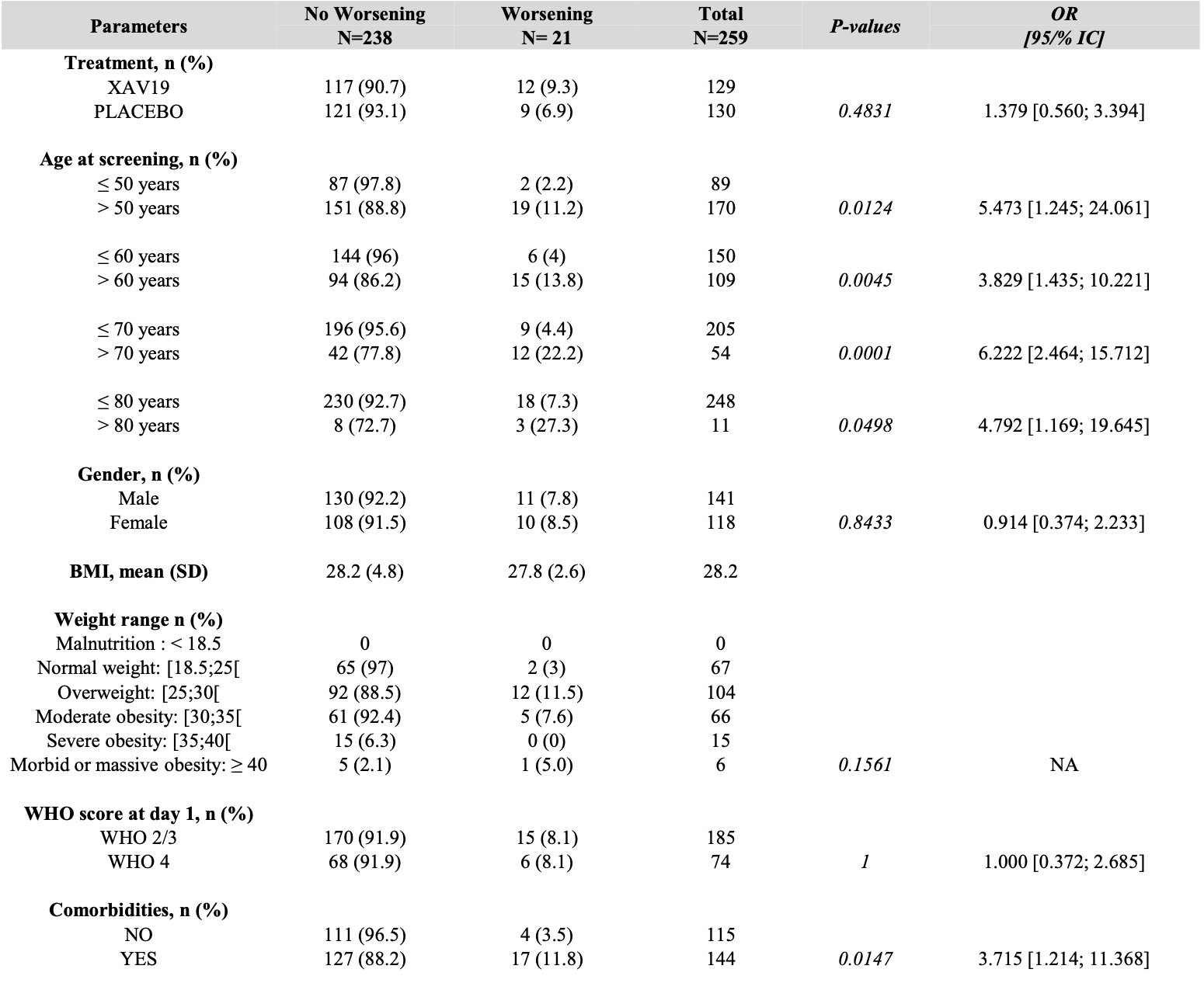
RCT 293 COVID-19 patients showing no significant difference in the primary endpoint (disease aggravation within 8 days) with XAV-19, a glyco-humanized swine polyclonal antibody. While XAV-19 showed no benefit for patients requiring oxygen therapy, it significantly reduced time to improvement for patients not requiring oxygen (7 days vs 14 days, p=0.0159).
|
risk of death, 0.7% higher, RR 1.01, p = 1.00, treatment 5 of 139 (3.6%), control 5 of 140 (3.6%).
|
|
risk of progression, 34.1% higher, RR 1.34, p = 0.48, treatment 12 of 117 (10.3%), control 9 of 121 (7.4%), odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Poulakou et al., 19 Oct 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, preprint, 10 authors, study period March 2021 - October 2022, trial NCT04928430 (history) (EUROXAV).
XAV-19 a Glyco-Humanized polyclonal antibody targeting SARS-CoV-2 accelerates the recovery of mild to moderate COVID-19 patients and keeps its neutralizing activity against Omicron and its subvariants
doi:10.1101/2023.10.09.23296726
Background: XAV-19 is a glyco-humanized swine polyclonal antibody targeting SARS-CoV-2. The safety and clinical efficacy of XAV-19 was investigated in patients with a WHO score of 2 to 4 in the WHO 7-point ordinal scale. The activity of XAV-19 against Omicron and its subvariants was assessed in vitro. Methods: A phase II/III, multicentric randomized double-blind placebo-controlled, clinical trial was conducted to evaluate the safety and clinical efficacy of XAV-19 in inpatients with COVID-19 requiring or not oxygen therapy and outpatients not requiring oxygen (EUROXAV trial, NCT04928430). Most patients were not vaccinated. The primary endpoint was the proportion of patients with an aggravation of COVID-19 within 8 days after treatment. Binding and neutralization of Omicron or its subvariants by XAV-19 was investigated by ELISA or with a whole virus neutralization assay. Results: Patients received either 150mg of XAV-19 (N=139) or placebo (N=140). Low enrolment forced the premature trial termination. XAV-19 was well tolerated. No difference in the primary endpoint, nor in the proportion with an improvement at day 8 (secondary endpoint) was observed between the 2 groups. For patients not requiring oxygen therapy, XAV-19 reduced the time to improvement significantly (7 days vs 14 days p=0.0159). Neutralizing activity against Omicron and BA.2, BA2.12.1, BA.4/5 and BQ1.1 subvariants was shown in vitro. Conclusions: XAV-19 did not reduce the aggravation in COVID-19 patients. While it did not bring any benefit to patients requiring oxygen, it reduced the time to improvement for patients not requiring oxygen (WHO score 2 or 3). These preliminary clinical data might indicate a therapeutic potential for patients with mild to moderate COVID-19 requiring supplementation with anti-SARS-CoV-2 neutralizing antibodies. .
References
Arora, Kempf, Nehlmeier, Schulz, Jäck et al., Omicron sublineage BQ.1.1 resistance to monoclonal antibodies, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00733-2
Brady, Gurijala, Huang, Hussain, Lingan et al., A guide to <scp>COVID</scp> -19 antiviral therapeutics: a summary and perspective of the antiviral weapons against <scp>SARS-CoV</scp> -2 infection, FEBS J, doi:10.1111/febs.16662
Callaway, Hastie, Schendel, Li, Yu et al., Bivalent intra-spike binding provides durability against emergent Omicron lineages: Results from a global consortium, Cell Rep, doi:10.1016/j.celrep.2023.112014
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, New England Journal of Medicine, doi:10.1056/nejmoa2001282
Casadevall, Focosi, SARS-CoV-2 variants resistant to monoclonal antibodies in immunocompromised patients constitute a public health concern, Journal of Clinical Investigation, doi:10.1172/JCI168603
Cunha, Stolet, Strauch, Pereira, Dumard et al., Polyclonal F(ab')2 fragments of equine antibodies raised against the spike protein neutralize SARS-CoV-2 variants with high potency, iScience, doi:10.1016/j.isci.2021.103315
Dewolf, Laracy, Perales, Kamboj, Van Den Brink et al., SARS-CoV-2 in immunocompromised individuals, Immunity, doi:10.1016/j.immuni.2022.09.006
Dhar, Sasmal, Varki, From "Serum Sickness" to "Xenosialitis": Past, Present, and Future Significance of the Nonhuman Sialic Acid Neu5Gc, Front Immunol, doi:10.3389/fimmu.2019.00807
Dong, Zost, Greaney, Starr, Dingens et al., Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail, Nat Microbiol, doi:10.1038/s41564-021-00972-2
Focosi, Maggi, Franchini, Mcconnell, Casadevall, Analysis of immune escape variants from antibody-based therapeutics against covid-19: A systematic review, Int J Mol Sci, doi:10.3390/ijms23010029
Focosi, Mcconnell, Casadevall, Cappello, Valdiserra et al., Monoclonal antibody therapies against SARS-CoV-2, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00311-5
Gaborit, Dailly, Vanhove, Josien, Lacombe et al., Pharmacokinetics and Safety of XAV-19, a Swine Glyco-humanized Polyclonal Anti-SARS-CoV-2 Antibody, for COVID-19-Related Moderate Pneumonia: a Randomized, Double-Blind, Placebo-Controlled, Phase IIa Study, Antimicrob Agents Chemother, doi:10.1128/AAC.01237-21
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Effect of Sotrovimab on Hospitalization or Death Among High-risk Patients With Mild to Moderate COVID-19, JAMA, doi:10.1001/jama.2022.2832
Gupta, Konnova, Smet, Berkell, Savoldi et al., Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments, Journal of Clinical Investigation, doi:10.1172/JCI166032
Huang, Mccreary, Bariola, Minnier, Wadas et al., Effectiveness of Casirivimab-Imdevimab and Sotrovimab During a SARS-CoV-2 Delta Variant Surge, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20957
Huo, Dijokaite-Guraliuc, Liu, Zhou, Ginn et al., A delicate balance between antibody evasion and ACE2 affinity for Omicron BA, Cell Rep, doi:10.1016/j.celrep.2022.111903
Kumari, Lu, Li, Huang, Hsu et al., A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies, J Biomed Sci, doi:10.1186/s12929-022-00852-9
Lopardo, Belloso, Nannini, Colonna, Sanguineti et al., RBD-specific polyclonal F(ab´)2 fragments of equine antibodies in patients with moderate to severe COVID-19 disease: A randomized, multicenter, double-blind, placebo-controlled, adaptive phase 2/3 clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100843
Nyberg, Ferguson, Nash, Webster, Flaxman et al., Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, The Lancet, doi:10.1016/S0140-6736(22)00462-7
O'brien, Forleo-Neto, Sarkar, Isa, Hou et al., Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.24939
Planas, Bruel, Staropoli, Guivel-Benhassine, Porrot et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies, Nat Commun, doi:10.1038/s41467-023-36561-6
Taiwo, Chew, Moser, Wohl, Daar et al., Phase 2 safety and antiviral activity of SAB-185, a novel polyclonal antibody therapy for nonhospitalized adults with COVID-19, J Infect Dis, doi:10.1093/infdis/jiad013
Turtle, Thorpe, Drake, Swets, Palmieri et al., Outcome of COVID-19 in hospitalised immunocompromised patients: An analysis of the WHO ISARIC CCP-UK prospective cohort study, PLoS Med, doi:10.1371/journal.pmed.1004086
Vanhove, Duvaux, Rousse, Royer, Evanno et al., High neutralizing potency of swine glyco-humanized polyclonal antibodies against SARS-CoV-2, Eur J Immunol, doi:10.1002/eji.202049072
Vanhove, Marot, So, Gaborit, Evanno et al., XAV-19, a Swine Glyco-Humanized Polyclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Domain, Targets Multiple Epitopes and Broadly Neutralizes Variants, Front Immunol, doi:10.3389/fimmu.2021.761250
Vanhove, Marot, So, Gaborit, Evanno et al., XAV-19, a Swine Glyco-Humanized Polyclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Domain, Targets Multiple Epitopes and Broadly Neutralizes Variants, Front Immunol, doi:10.3389/fimmu.2021.761250
Widyasari, Kim, A Review of the Currently Available Antibody Therapy for the Treatment of Coronavirus Disease 2019 (COVID-19), Antibodies, doi:10.3390/antib12010005
DOI record:
{
"DOI": "10.1101/2023.10.09.23296726",
"URL": "http://dx.doi.org/10.1101/2023.10.09.23296726",
"abstract": "<jats:p>Background: XAV-19 is a glyco-humanized swine polyclonal antibody targeting SARS-CoV-2. The safety and clinical efficacy of XAV-19 was investigated in patients with a WHO score of 2 to 4 in the WHO 7-point ordinal scale. The activity of XAV-19 against Omicron and its subvariants was assessed in vitro. Methods: A phase II/III, multicentric randomized double-blind placebo-controlled, clinical trial was conducted to evaluate the safety and clinical efficacy of XAV-19 in inpatients with COVID-19 requiring or not oxygen therapy and outpatients not requiring oxygen (EUROXAV trial,<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04928430\">NCT04928430</jats:ext-link>). Most patients were not vaccinated. The primary endpoint was the proportion of patients with an aggravation of COVID-19 within 8 days after treatment. Binding and neutralization of Omicron or its subvariants by XAV-19 was investigated by ELISA or with a whole virus neutralization assay. Results: Patients received either 150mg of XAV-19 (N=139) or placebo (N=140). Low enrolment forced the premature trial termination. XAV-19 was well tolerated. No difference in the primary endpoint, nor in the proportion with an improvement at day 8 (secondary endpoint) was observed between the 2 groups. For patients not requiring oxygen therapy, XAV-19 reduced the time to improvement significantly (7 days vs 14 days p=0.0159). Neutralizing activity against Omicron and BA.2, BA2.12.1, BA.4/5 and BQ1.1 subvariants was shown in vitro. Conclusions: XAV-19 did not reduce the aggravation in COVID-19 patients. While it did not bring any benefit to patients requiring oxygen, it reduced the time to improvement for patients not requiring oxygen (WHO score 2 or 3). These preliminary clinical data might indicate a therapeutic potential for patients with mild to moderate COVID-19 requiring supplementation with anti-SARS-CoV-2 neutralizing antibodies.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
10,
19
]
]
},
"author": [
{
"affiliation": [],
"family": "Poulakou",
"given": "Garyfallia",
"sequence": "first"
},
{
"affiliation": [],
"family": "Royer",
"given": "Pierre-Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evgeniev",
"given": "Nikolai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evanno",
"given": "Gwenaëlle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shneiker",
"given": "Françoise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcelin",
"given": "Anne-Geneviève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vanhove",
"given": "Bernard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duvaux",
"given": "Odile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marot",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calvez",
"given": "Vincent",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T20:05:20Z",
"timestamp": 1697745920000
},
"deposited": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T20:05:20Z",
"timestamp": 1697745920000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T05:35:20Z",
"timestamp": 1697780120934
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10,
19
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2023.10.09.23296726",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
10,
19
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2023,
10,
19
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2023.10.09.23296726"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "XAV-19 a Glyco-Humanized polyclonal antibody targeting SARS-CoV-2 accelerates the recovery of mild to moderate COVID-19 patients and keeps its neutralizing activity against Omicron and its subvariants.",
"type": "posted-content"
}