Feb 21 |
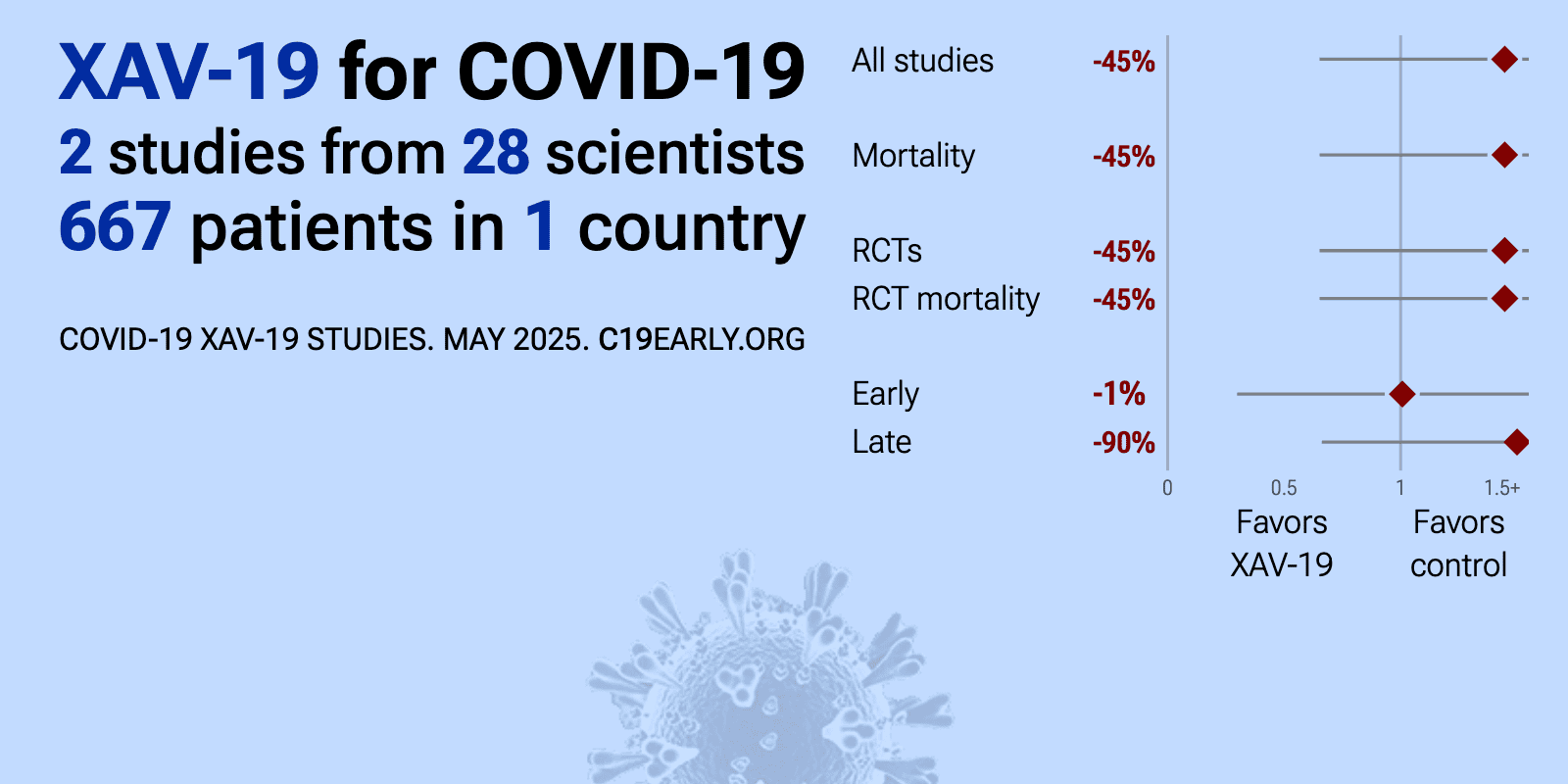
Meta-analysis of XAV-19 studies | |
| Meta-analysis of XAV-19 studies | ||
Oct 20 2023 |
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad525 | Effect of Swine Glyco-humanized Polyclonal Neutralizing Antibody on Survival and Respiratory Failure in Patients Hospitalized With Severe COVID-19: A Randomized, Placebo-Controlled Trial |
| 90% higher mortality (p=0.29), 106% higher ventilation (p=0.03), 5% higher ICU admission (p=0.9), and 5% higher progression (p=0.82). RCT 398 hospitalized patients with severe COVID-19 requiring low-flow oxygen therapy showing no significant difference in death or respiratory failure through day 15 with XAV-19 (a swine glyco-humanized polyclonal neutralizing antibody) c.. | ||
Oct 19 2023 |
et al., medRxiv, doi:10.1101/2023.10.09.23296726 | XAV-19 a Glyco-Humanized polyclonal antibody targeting SARS-CoV-2 accelerates the recovery of mild to moderate COVID-19 patients and keeps its neutralizing activity against Omicron and its subvariants |
| 1% higher mortality (p=1) and 34% higher progression (p=0.48). RCT 293 COVID-19 patients showing no significant difference in the primary endpoint (disease aggravation within 8 days) with XAV-19, a glyco-humanized swine polyclonal antibody. While XAV-19 showed no benefit for patients requiring oxygen.. | ||