Clinical Outcome of Patients with COVID-19 Pneumonia Treated with Corticosteroids and Colchicine in Colombia
et al., Research Square, doi:10.21203/rs.3.rs-94922/v1, Oct 2020
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
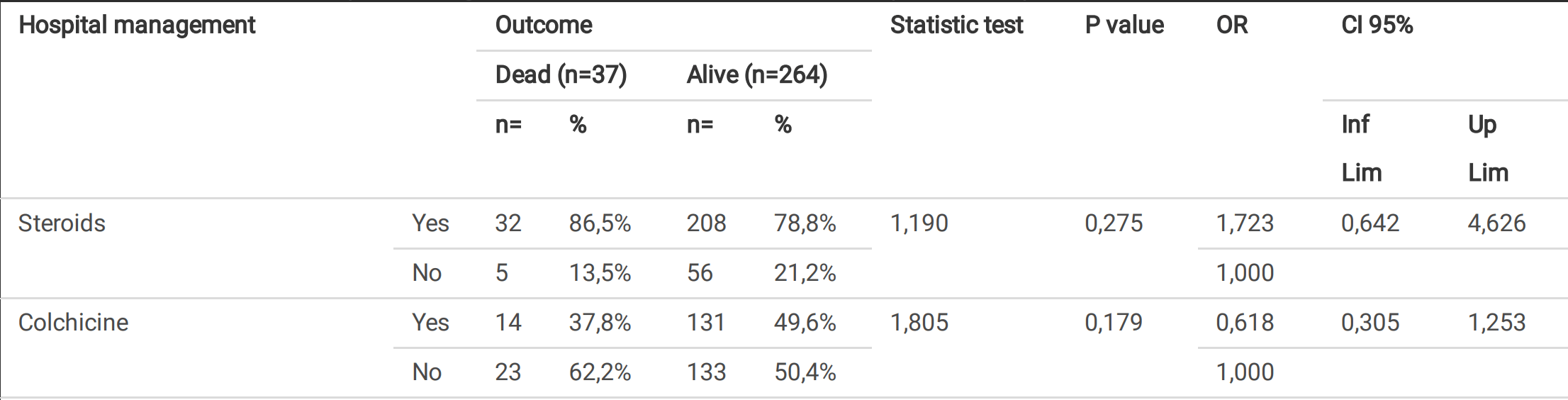
Retrospective 301 pneumonia patients in Colombia showing lower mortality with colchicine treatment.
|
risk of death, 34.5% lower, RR 0.65, p = 0.18, treatment 14 of 145 (9.7%), control 23 of 156 (14.7%), NNT 20, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pinzón et al., 23 Oct 2020, retrospective, Colombia, preprint, 9 authors, dosage 1mg days 1-14.
Clinical Outcome of Patients with COVID-19 Pneumonia Treated with Corticosteroids and Colchicine in Colombia
doi:10.21203/rs.3.rs-94922/v1
Background: To date, there is no speci c antiviral therapy for severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) that causes Coronavirus disease 2019 . Since there is no speci c therapy against SARS-CoV2, current efforts aim to prevent contagion through public health measures and develop a protective vaccine. While waiting for the latter, it is necessary to evaluate the drugs that at least, in initial studies, suggested some degree of utility in the management of Covid-19 or its complications. The Objective of the study was to describe the clinical manifestations and outcomes of patients with severe Covid-19 Pneumonia treated with corticosteroids and colchicine. Materials and Methods: A cross sectional study of 301 adult patients with Covid-19 Pneumonia con rmed by Real-Time Polymerase Chain Reaction for SARS-CoV2 (RT-PCR SARS-CoV2), Berlin protocol, who required hospitalization in three hospitals in Antioquia, Colombia. Patients were treated according to the institutional protocol (from March 20, 2020 to June 30, 2020) with corticosteroid if the patient required supplemental oxygen. From July 1, 2020, the management protocol changed with the addition of colchicine to all patients admitted to the institutions. The treatment was supervised and monitored by the same specialist in infectology of the institutions. We describe the clinical manifestations and outcomes of the patients who received these treatments. The patient's information was analyzed according to the outcome of interest (alive/dead) with univariate, bivariate, and multivariate measures to adjust the variables that presented statistical association. Results: All patients had pneumonia documented by chest computed tomography with ground glass images and presented an alveolar pressure / inspired oxygen fraction (PaFi) less than 300. 240 (79.7%) of patients received corticosteroids, and 145 (48.2%) also received colchicine; of these, 14 (9.6%) died vs. 23 (14.7%) of those who did not receive it. Hospital mortality due to severe Covid-19 Pneumonia was 12.3% in three hospitals in Colombia. Conclusions: Treatment with corticosteroids and colchicine for managing patients with severe Covid-19 pneumonia was associated with low mortality at the hospital level. Randomized, placebo-controlled studies are required to evaluate the effect of corticosteroids and colchicine on complications or death from Covid-19. Jul 28.
Authors' contributions
Ethics approval The study was approved by the ethics committees of Clínica Medellín (number 04-2020), Nueva Clínica Sagrado Corazón, and Clínica Panamericana. Informed consent was obtained from the study participants.
Consent for participate We wish to submit the manuscript for publication in Annals of Clinical Microbiology and Antimicrobials®, and the manuscript is not currently under consideration for publication in another journal.
Competing interest The authors declare that they have no competing interests
References
Arabi, Deeb, Al-Hameed, Macrolides in critically ill patients with Middle East Respiratory Syndrome, Int J Infect Dis, doi:10.1016/j.ijid.2019.01.041
Colson, Rolain, Lagier, Brouqui, Raoult, Chloroquine and hydroxychloroquine as available weapons to ght COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105932
De Wit, Feldmann, Cronin, Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection, Proc Natl Acad Sci U S A, doi:10.1073/pnas.1922083117
Deftereos, Giannopoulos, Vrachatis, Gerasimos, Giotaki et al., Effect of Colchicine vs Standard Care on Cardiac and In ammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13136
Dong, Hu, Gao, Discovering drugs to treat coronavirus disease 2019 (COVID-19), Drug Discov Ther, doi:10.5582/ddt.2020.01012
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105949
Geleris, Sun, Platt, Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2012410
Jesús, Carlos, Domingo, Dexamethasone Treatment for the Acute Respiratory Distress Syndrome: A Multicentre, Randomised Controlled Trial, Lancet Respir Med, doi:10.1016/S2213-2600(19)30417-5
Jin, Cai, Cheng, A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil Med Res, doi:10.1186/s40779-020-0233-6
Karagiannidis, Mostert, Hentschker, Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study, Lancet Respir Med, doi:10.1016/S2213-2600(20)30316-7
Lee, Gardner, Porter, Current concepts in the diagnosis and management of cytokine release syndrome, Blood
Lupia, Scabini, Mornese Pinna, novel coronavirus (2019-nCoV) outbreak: A new challenge, J. Glob. Antimicrob. Resist
Mahase, Coronavirus COVID-19 has killed more people than SARS and MERS combined, despite lower case fatality rate, BMJ, doi:10.1136/bmj.m641
Mansouri, Marjani, Tabarsi, Garnier, Mansouri, Successful Treatment of Covid-19 Associated Cytokine Release Syndrome with Colchicine. A Case Report and Review of Literature, Immunol Invest, doi:10.1080/08820139.2020.1789655
Martinon, Pétrilli, Mayor, Tardivel, Tschopp, Gout associated uric acid crystals activate the NALP3 in ammasome, Nature
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Recovery Collaborative Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19 -Preliminary Report, N Engl J Med, doi:10.1056/NEJMoa2021436
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet, doi:10.1016/S0140-6736(20)30317-2
Scarsi, Piantoni, Colombo, Airó, Richini et al., Association between treatment with colchicine and improved survival in a singlecentre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217712
Wu Chaomin, Xiaoyan, Yanping, Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China, JAMA Intern Med, doi:10.1001/jamainternmed.2020.0994
Yao, Qian, Zhu, Wang, Wang, A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option, J Med Virol, doi:10.1002/jmv.25729
Yao, Ye, Zhang, Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
Yao, Ye, Zhang, Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin Infect Dis, doi:10.1093/cid/ciaa237
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.21203/rs.3.rs-94922/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-94922/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p><jats:bold>Background:</jats:bold> To date, there is no specific antiviral therapy for severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) that causes Coronavirus disease 2019 (Covid-19). Since there is no specific therapy against SARS-CoV2, current efforts aim to prevent contagion through public health measures and develop a protective vaccine. While waiting for the latter, it is necessary to evaluate the drugs that at least, in initial studies, suggested some degree of utility in the management of Covid-19 or its complications.The Objective of the study was to describe the clinical manifestations and outcomes of patients with severe Covid-19 Pneumonia treated with corticosteroids and colchicine.<jats:bold>Materials and Methods:<jats:italic> </jats:italic></jats:bold>A cross sectional study of 301 adult patients with Covid-19 Pneumonia confirmed by Real-Time Polymerase Chain Reaction for SARS-CoV2 (RT-PCR SARS-CoV2), Berlin protocol, who required hospitalization in three hospitals in Antioquia, Colombia. Patients were treated according to the institutional protocol (from March 20, 2020 to June 30, 2020) with corticosteroid if the patient required supplemental oxygen. From July 1, 2020, the management protocol changed with the addition of colchicine to all patients admitted to the institutions. The treatment was supervised and monitored by the same specialist in infectology of the institutions. We describe the clinical manifestations and outcomes of the patients who received these treatments. The patient’s information was analyzed according to the outcome of interest (alive/dead) with univariate, bivariate, and multivariate measures to adjust the variables that presented statistical association.<jats:bold>Results:<jats:italic> </jats:italic></jats:bold>All patients had pneumonia documented by chest computed tomography with ground glass images and presented an alveolar pressure / inspired oxygen fraction (PaFi) less than 300. 240 (79.7%) of patients received corticosteroids, and 145 (48.2%) also received colchicine; of these, 14 (9.6%) died vs. 23 (14.7%) of those who did not receive it. Hospital mortality due to severe Covid-19 Pneumonia was 12.3% in three hospitals in Colombia.<jats:bold>Conclusions:<jats:italic> </jats:italic></jats:bold>Treatment with corticosteroids and colchicine for managing patients with severe Covid-19 pneumonia was associated with low mortality at the hospital level. Randomized, placebo-controlled studies are required to evaluate the effect of corticosteroids and colchicine on complications or death from Covid-19.</jats:p>",
"accepted": {
"date-parts": [
[
2020,
10,
19
]
]
},
"author": [
{
"affiliation": [
{
"name": "Clinica Medellin"
}
],
"family": "Pinzón",
"given": "Miguel Alejandro",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Universidad CES"
}
],
"family": "Arango",
"given": "Doris Cardona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Medellín"
}
],
"family": "Betancur",
"given": "Juan Felipe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Medellin"
}
],
"family": "Holguín",
"given": "Héctor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universidad CES"
}
],
"family": "Arias",
"given": "Carolina Arias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Medellín"
}
],
"family": "Muñoz",
"given": "Bernardo Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Medellin"
}
],
"family": "Amarillo",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Medellín"
}
],
"family": "Llano",
"given": "Juan Felipe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Medellin"
}
],
"family": "Montoya",
"given": "Pablo",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
10,
23
]
],
"date-time": "2020-10-23T14:02:27Z",
"timestamp": 1603461747000
},
"deposited": {
"date-parts": [
[
2022,
7,
29
]
],
"date-time": "2022-07-29T00:00:29Z",
"timestamp": 1659052829000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
3,
3
]
],
"date-time": "2024-03-03T14:27:54Z",
"timestamp": 1709476074943
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 7,
"issued": {
"date-parts": [
[
2020,
10,
23
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
23
]
],
"date-time": "2020-10-23T00:00:00Z",
"timestamp": 1603411200000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-94922/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-94922/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2020,
10,
23
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2020,
10,
23
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1186/s12941-021-00460-9",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-94922/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Clinical Outcome of Patients with COVID-19 Pneumonia Treated with Corticosteroids and Colchicine in Colombia",
"type": "posted-content"
}
