Effectiveness and Safety of Regdanvimab in Patients With Mild-To-Moderate COVID-19: A Retrospective Cohort Study
et al., Journal of Korean Medical Science, doi:10.3346/jkms.2022.37.e102, Mar 2022
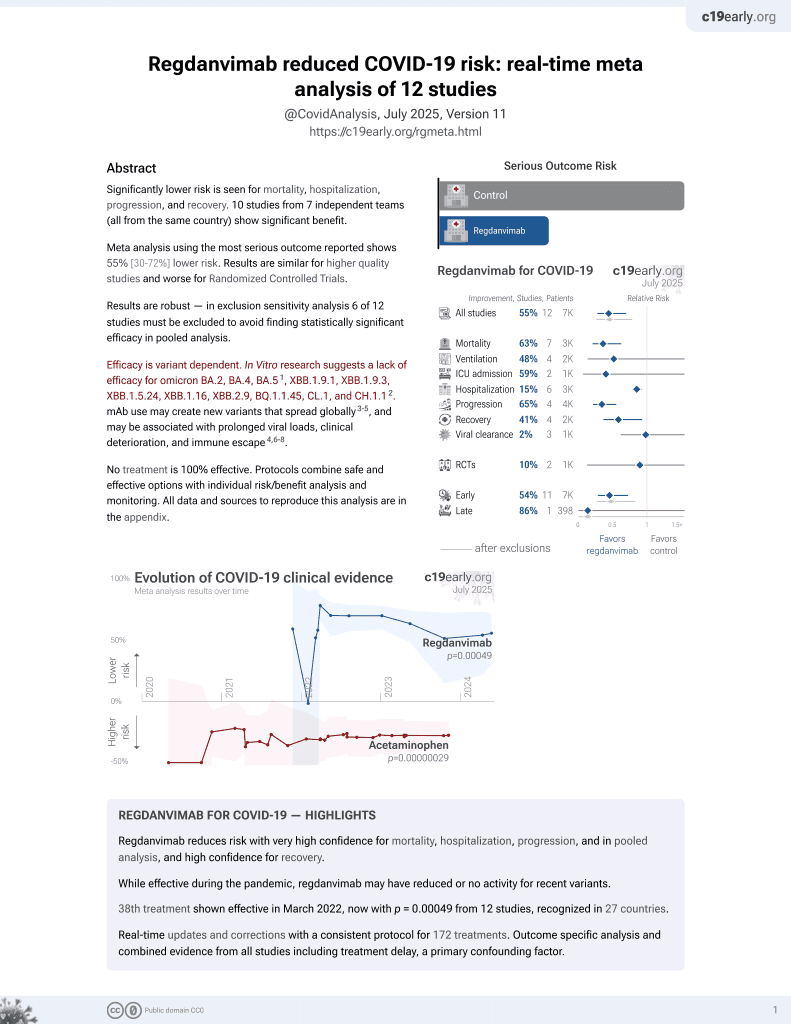
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
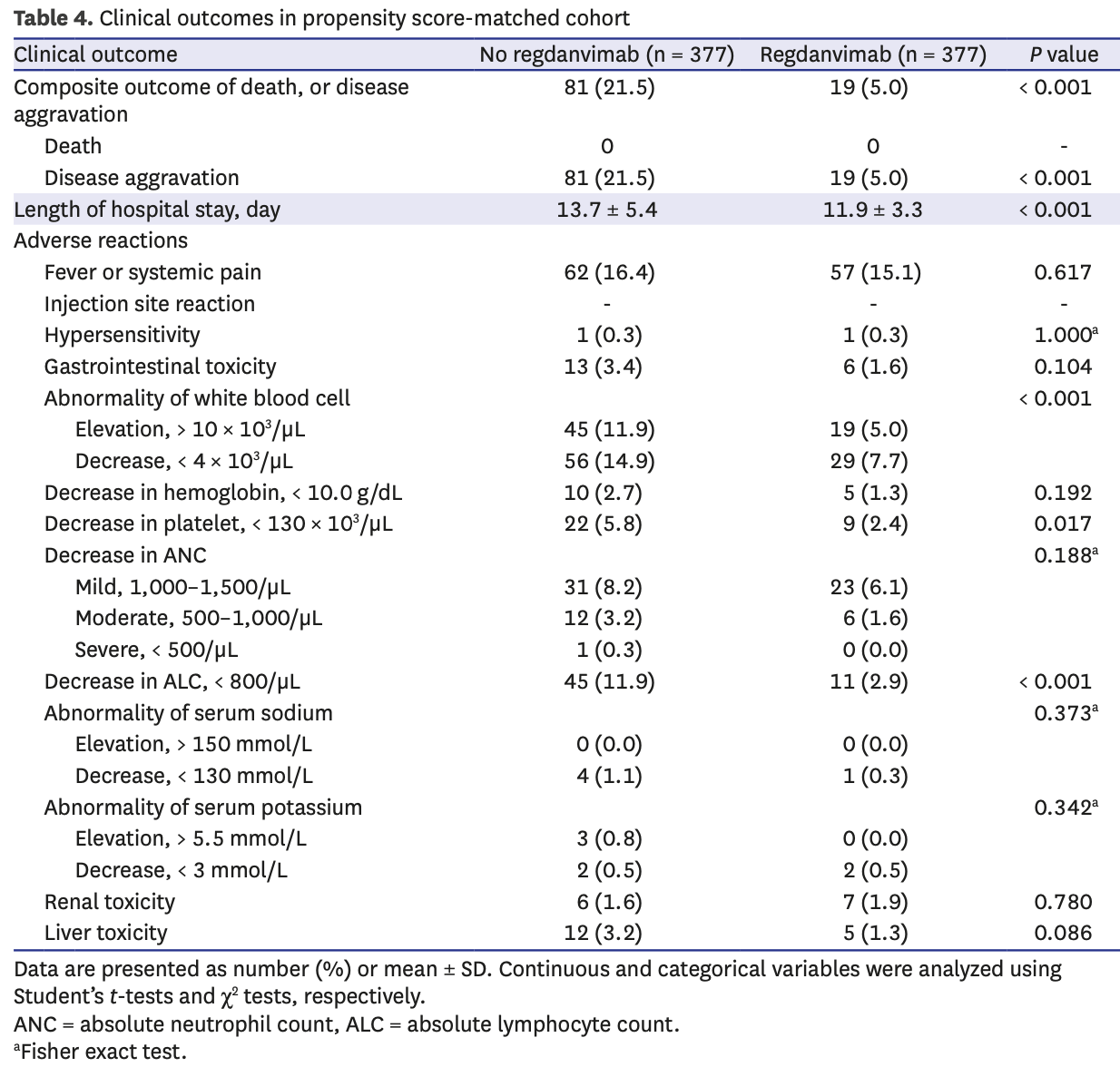
Retrospective propensity score matched analysis of 970 high-risk mild-moderate COVID-19 patients in South Korea, showing regdanvimab significantly reduced risk of disease progression or death by 77% compared to standard care alone. No deaths occurred in either group. Regdanvimab also significantly shortened hospital stay and reduced hematological adverse events.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending regdanvimab also recommended them, or
because the patient seeking out regdanvimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.54, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.15.
|
risk of progression, 79.4% lower, RR 0.21, p < 0.001, treatment 19 of 377 (5.0%), control 81 of 377 (21.5%), NNT 6.1, adjusted per study, odds ratio converted to relative risk, disease aggravation or death, propensity score matching, multivariable.
|
|
hospitalization time, 13.1% lower, relative time 0.87, p < 0.001, treatment mean 11.9 (±3.3) n=377, control mean 13.7 (±5.4) n=377, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Park et al., 29 Mar 2022, retrospective, South Korea, peer-reviewed, 5 authors, study period 1 December, 2020 - 16 April, 2021.
Contact: mdhuh@hanmail.net.
Effectiveness and Safety of Regdanvimab in Patients With Mild-To-Moderate COVID-19: A Retrospective Cohort Study
Journal of Korean Medical Science, doi:10.3346/jkms.2022.37.e102
Background: Regdanvimab has decreased the time to clinical recovery from coronavirus disease 2019 (COVID-19) and lowered the rate of oxygen therapy according to the results from phase 2/3 randomized controlled trial. More information is needed about the effects and safety of regdanvimab. Methods: We analyzed data for patients with high-risk mild or moderate COVID-19 being admitted to Busan Medical Center between December 1, 2020 and April 16, 2021. A propensity score (PS) matched analysis was conducted to compare patients treated with and without regdanvimab. The primary outcome was in-hospital death or disease aggravation which means the need for oxygen therapy (low-or high-flow oxygen therapy and mechanical ventilation) and secondary outcomes comprised the length of hospital stay and adverse reactions. Results: Among 1,617 selected patients, 970 (60.0%) were indicated for regdanvimab. Of these, 377 (38.9%) were administered with regdanvimab. Among a 1:1 PS-matched cohort of 377 patients each treated with and without regdanvimab, 19 (5%) and 81 (21.5%) reached the composite outcome of death, or disease aggravation, respectively (absolute risk difference, -16.4%; 95% confidence interval [CI], -21.1, -11.7; relative risk difference, 76.5%; P < 0.001). Regdanvimab significantly reduced the composite outcome of death, or disease aggravation in univariate (odds ratio [OR], 0.194; 95% CI, 0.112-0.320; P < 0.001) and multivariableadjusted analyses (OR, 0.169; 95% CI, 0.095-0.289; P < 0.001). The hospital stay was shorter for the group with than without regdanvimab. Some hematological adverse reactions were more frequent in the group without regdanvimab, but other adverse reactions did not significantly differ between the groups. Conclusion: Regdanvimab was associated with a significantly lower risk of disease aggravation without increasing adverse reactions.
SUPPLEMENTARY MATERIALS Supplementary Table 1 Missing baseline laboratory data in propensity score-matched cohort
Click here to view
Supplementary Table 2 Associations between regdanvimab and primary endpoint in PS-matched and overall cohorts
Click here to view
Supplementary Table 3
Clinical outcomes in overall cohort Click here to view Supplementary Fig. 1 Distribution of covariates before and after propensity matching study patients who were and were not treated with regdanvimab. Click here to view Supplementary Fig. 2 Standardized mean differences in unadjusted and adjusted cohorts.
Click here to view
References
Baum, Fulton, Wloga, Copin, Pascal et al., Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science, doi:10.1126/science.abd0831
Chow, Khanna, Kethireddy, Yamane, Levine et al., Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019, Anesth Analg, doi:10.1213/ANE.0000000000005292
Daniels, Sitapati, Zhang, Zou, Bui et al., Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients, Am J Cardiol, doi:10.1016/j.amjcard.2020.09.012
Eom, Ison, Streinu-Cercel, Săndulescu, Preotescu et al., Efficacy and safety of CT-P59 plus standard of care: a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection, Res Sq, doi:10.21203/rs.3.rs-296518/v1
Fosbøl, Butt, Østergaard, Andersson, Selmer et al., Association of angiotensinconverting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality, JAMA, doi:10.1001/jama.2020.11301
Gebhard, Regitz-Zagrosek, Neuhauser, Morgan, Klein, Impact of sex and gender on COVID-19 outcomes in Europe, Biol Sex Differ, doi:10.1186/s13293-020-00304-9
Gupta, Madhavan, Poterucha, Defilippis, Hennessey et al., Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19, Nat Commun, doi:10.1038/s41467-021-21553-1
Hj, Kim, Kang, Yang, Lee, Prediction of COVID-19-related mortality and 30-day and 60day survival probabilities using a nomogram, J Korean Med Sci, doi:10.3346/jkms.2021.36.e248
Hurt, Wheatley, Neutralizing antibody therapeutics for COVID-19, Viruses, doi:10.3390/v13040628
Jahanshahlu, Rezaei, Monoclonal antibody as a potential anti-COVID-19, Biomed Pharmacother, doi:10.1016/j.biopha.2020.110337
Jia, Gong, Will mutations in the spike protein of SARS-CoV-2 lead to the failure of COVID-19 vaccines?, J Korean Med Sci, doi:10.3346/jkms.2021.36.e124
Kim, Ryoo, Huh, Joo, Kim et al., Revised Korean Society of Infectious Diseases/ National Evidence-based Healthcarea collaborating agency guidelines on the treatment of patients with COVID-19, Infect Chemother, doi:10.3947/ic.2021.0303
Kim, Ryu, Lee, Kim, Seo et al., A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat Commun, doi:10.1038/s41467-020-20602-5
Lam, Chow, Vo, Hou, Li et al., Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin ii receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome, J Infect Dis, doi:10.1093/infdis/jiaa447
Lee, Choe, Jeong, Kim, Kim et al., Importation and transmission of SARS-CoV-2 B.1.1.529 (Omicron) variant of concern in Korea, November 2021, J Korean Med Sci, doi:10.3346/jkms.2021.36.e346
Li, Xu, Yu, Wang, Tao et al., Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan, J Allergy Clin Immunol, doi:10.1016/j.jaci.2020.04.006
Orders, An EUA for sotrovimab for treatment of COVID-19, Med Lett Drugs Ther
Osborne, Veigulis, Arreola, Mahajan, Röösli et al., Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration, PLoS One, doi:10.1371/journal.pone.0246825
Pastor-Nieto, Checa-Díaz, González-Muñoz, Martín-Fuentes, Vergara-Sánchez et al., Prior treatment with immunosuppressants among COVID-19 inpatients at one hospital in Spain, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16798
Prabakaran, Zhu, Xiao, Biragyn, Dimitrov et al., Potent human monoclonal antibodies against SARS CoV, Nipah and Hendra viruses, Expert Opin Biol Ther, doi:10.1517/14712590902763755
Ryu, Hong, Lim, Cho, Hwang et al., Clinical features of adult COVID-19 patients without risk factors before and after the nationwide SARS-CoV-2 B.1.617.2 (Delta)-variant outbreak in Korea: experience from Gyeongsangnam-do, J Korean Med Sci, doi:10.3346/jkms.2021.36.e341
Ryu, Song, Kim, Kim, Kim et al., Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant, doi:10.1016/j.bbrc.2021.06.016
Saphire, Schendel, Gunn, Milligan, Alter, Antibody-mediated protection against Ebola virus, Nat Immunol, doi:10.1038/s41590-018-0233-9
Scully, Haverfield, Ursin, Tannenbaum, Klein, Considering how biological sex impacts immune responses and COVID-19 outcomes, Nat Rev Immunol, doi:10.1038/s41577-020-0348-8
Wang, Li, Drabek, Okba, Van Haperen et al., A human monoclonal antibody blocking SARS-CoV-2 infection, Nat Commun, doi:10.1038/s41467-020-16256-y
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Yi, Kim, Choe, Hong, Choi et al., SARS-CoV-2 delta variant breakthrough infection and onward secondary transmission in household, J Korean Med Sci, doi:10.3346/jkms.2022.37.e12
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
DOI record:
{
"DOI": "10.3346/jkms.2022.37.e102",
"ISSN": [
"1011-8934",
"1598-6357"
],
"URL": "http://dx.doi.org/10.3346/jkms.2022.37.e102",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2021-11-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"value": "2022-03-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published online",
"name": "published_online",
"value": "2022-03-29"
},
{
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Korean Academy of Medical Sciences."
},
{
"explanation": {
"URL": "https://creativecommons.org/licenses/by-nc/4.0/"
},
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "License",
"name": "license",
"value": "This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7016-5124",
"affiliation": [
{
"name": "College of Pharmacy, Pusan National University, Busan, Korea."
}
],
"authenticated-orcid": false,
"family": "Park",
"given": "Susin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0299-5131",
"affiliation": [
{
"name": "College of Pharmacy, Pusan National University, Busan, Korea."
}
],
"authenticated-orcid": false,
"family": "Je",
"given": "Nam Kyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7230-3540",
"affiliation": [
{
"name": "Division of Pulmonology, Department of Internal Medicine, Busan Medical Center, Busan, Korea."
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Dong Wan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4986-4072",
"affiliation": [
{
"name": "Division of Pulmonology, Department of Internal Medicine, Busan Medical Center, Busan, Korea."
}
],
"authenticated-orcid": false,
"family": "Park",
"given": "Miran",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9534-2921",
"affiliation": [
{
"name": "Division of Pulmonology, Department of Internal Medicine, Busan Medical Center, Busan, Korea."
}
],
"authenticated-orcid": false,
"family": "Heo",
"given": "Jeonghun",
"sequence": "additional"
}
],
"container-title": "Journal of Korean Medical Science",
"container-title-short": "J Korean Med Sci",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"jkms.org"
]
},
"created": {
"date-parts": [
[
2022,
3,
28
]
],
"date-time": "2022-03-28T23:51:23Z",
"timestamp": 1648511483000
},
"deposited": {
"date-parts": [
[
2022,
4,
3
]
],
"date-time": "2022-04-03T23:59:51Z",
"timestamp": 1649030391000
},
"indexed": {
"date-parts": [
[
2023,
10,
25
]
],
"date-time": "2023-10-25T05:49:20Z",
"timestamp": 1698212960639
},
"is-referenced-by-count": 6,
"issue": "13",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "13",
"published-online": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
}
],
"link": [
{
"URL": "https://jkms.org/pdf/10.3346/jkms.2022.37.e102",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://jkms.org/DOIx.php?id=10.3346/jkms.2022.37.e102",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://jkms.org/DOIx.php?id=10.3346/jkms.2022.37.e102",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1898",
"original-title": [],
"prefix": "10.3346",
"published": {
"date-parts": [
[
2022
]
]
},
"published-online": {
"date-parts": [
[
2022
]
]
},
"publisher": "Korean Academy of Medical Sciences",
"reference": [
{
"DOI": "10.1038/s41586-020-2012-7",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"issue": "7798",
"journal-title": "Nature",
"key": "10.3346/jkms.2022.37.e102_ref1",
"volume": "579",
"year": "2020"
},
{
"key": "10.3346/jkms.2022.37.e102_ref2",
"unstructured": "World Health Organization. Director-general's remarks at the media briefing on 2019-nCoV on 11 February 2020. Updated 2020. Accessed November 1, 2021. http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020"
},
{
"key": "10.3346/jkms.2022.37.e102_ref3",
"unstructured": "Our World in Data. Statistics and research coronavirus (COVID-19) vaccinations. Updated 2021. Accessed November 1, 2021. https://ourworldindata.org/covid-vaccinations"
},
{
"key": "10.3346/jkms.2022.37.e102_ref4",
"unstructured": "World Health Organization. COVID-19 weekly epidemiological update. Updated 2021. Accessed November 1, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---26-october-2021"
},
{
"key": "10.3346/jkms.2022.37.e102_ref5",
"unstructured": "Johns Hopkins Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Updated 2021. Accessed November 1, 2021. https://coronavirus.jhu.edu/map.html"
},
{
"key": "10.3346/jkms.2022.37.e102_ref6",
"unstructured": "World Health Organization. Tracking SARS-CoV-2 variants. Updated 2021. Accessed November 1, 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/"
},
{
"DOI": "10.3346/jkms.2022.37.e12",
"author": "Yi",
"doi-asserted-by": "crossref",
"first-page": "e12",
"issue": "1",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2022.37.e102_ref7",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.3346/jkms.2021.36.e346",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e346",
"issue": "50",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2022.37.e102_ref8",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3346/jkms.2021.36.e124",
"author": "Jia",
"doi-asserted-by": "crossref",
"first-page": "e124",
"issue": "18",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2022.37.e102_ref9",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3346/jkms.2021.36.e341",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "e341",
"issue": "49",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2022.37.e102_ref10",
"volume": "36",
"year": "2021"
},
{
"key": "10.3346/jkms.2022.37.e102_ref11",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline. Updated 2022. Accessed November 1, 2021. https://www.who.int/publications/i/item/therapeutics-and-covid-19-living-guideline"
},
{
"key": "10.3346/jkms.2022.37.e102_ref12",
"unstructured": "National Institutes of Health. COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated 2021. Accessed November 1, 2021. https://www.covid19treatmentguidelines.nih.gov/"
},
{
"DOI": "10.1038/s41467-020-16256-y",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2251",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.3346/jkms.2022.37.e102_ref13",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2020.110337",
"author": "Jahanshahlu",
"doi-asserted-by": "crossref",
"first-page": "110337",
"journal-title": "Biomed Pharmacother",
"key": "10.3346/jkms.2022.37.e102_ref14",
"volume": "129",
"year": "2020"
},
{
"DOI": "10.1038/s41590-018-0233-9",
"author": "Saphire",
"doi-asserted-by": "crossref",
"first-page": "1169",
"issue": "11",
"journal-title": "Nat Immunol",
"key": "10.3346/jkms.2022.37.e102_ref15",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1517/14712590902763755",
"author": "Prabakaran",
"doi-asserted-by": "crossref",
"first-page": "355",
"issue": "3",
"journal-title": "Expert Opin Biol Ther",
"key": "10.3346/jkms.2022.37.e102_ref16",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "288",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.3346/jkms.2022.37.e102_ref17",
"volume": "12",
"year": "2021"
},
{
"author": "Eom",
"journal-title": "Res Sq",
"key": "10.3346/jkms.2022.37.e102_ref18"
},
{
"key": "10.3346/jkms.2022.37.e102_ref19",
"unstructured": "Ministry of Food and Drug Safety. Approval of Regkirona® Inj., an antibody treatment for COVID-19. Updated 2021. Accessed November 1, 2021. https://www.mfds.go.kr/brd/m_99/view.do?seq=45029&srchFr=&srchTo=&srchWord=&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C1009&page=2"
},
{
"key": "10.3346/jkms.2022.37.e102_ref20",
"unstructured": "Ministry of Food and Drug Safety. Pharmaceutical product information: Regkirona® Inj. 960 mg (regdanvimab). Updated 2021. Accessed November 1, 2021. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=202101124"
},
{
"key": "10.3346/jkms.2022.37.e102_ref21",
"unstructured": "European Medicines Agency. EMA issues advice on use of regdanvimab for treating COVID-19. Updated 2021. Accessed November 1, 2021. https://www.ema.europa.eu/en/news/ema-issues-advice-use-regdanvimab-treating-covid-19"
},
{
"key": "10.3346/jkms.2022.37.e102_ref22",
"unstructured": "Ministry of Food and Drug Safety. Official approval of Regkirona® Inj., an antibody treatment for COVID-19. Updated 2021. Accessed November 1, 2021. https://www.mfds.go.kr/brd/m_99/view.do?seq=45778&srchFr=&srchTo=&srchWord=&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C1009&page=1"
},
{
"DOI": "10.3947/ic.2021.0303",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "166",
"issue": "1",
"journal-title": "Infect Chemother",
"key": "10.3346/jkms.2022.37.e102_ref23",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiaa447",
"author": "Lam",
"doi-asserted-by": "crossref",
"first-page": "1256",
"issue": "8",
"journal-title": "J Infect Dis",
"key": "10.3346/jkms.2022.37.e102_ref24",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.11301",
"author": "Fosbøl",
"doi-asserted-by": "crossref",
"first-page": "168",
"issue": "2",
"journal-title": "JAMA",
"key": "10.3346/jkms.2022.37.e102_ref25",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21553-1",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1325",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.3346/jkms.2022.37.e102_ref26",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.amjcard.2020.09.012",
"author": "Daniels",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Am J Cardiol",
"key": "10.3346/jkms.2022.37.e102_ref27",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0246825",
"author": "Osborne",
"doi-asserted-by": "crossref",
"first-page": "e0246825",
"issue": "2",
"journal-title": "PLoS One",
"key": "10.3346/jkms.2022.37.e102_ref28",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"author": "Chow",
"doi-asserted-by": "crossref",
"first-page": "930",
"issue": "4",
"journal-title": "Anesth Analg",
"key": "10.3346/jkms.2022.37.e102_ref29",
"volume": "132",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.04.006",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "110",
"issue": "1",
"journal-title": "J Allergy Clin Immunol",
"key": "10.3346/jkms.2022.37.e102_ref30",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16798",
"author": "Pastor-Nieto",
"doi-asserted-by": "crossref",
"first-page": "e760",
"issue": "12",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "10.3346/jkms.2022.37.e102_ref31",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0348-8",
"author": "Scully",
"doi-asserted-by": "crossref",
"first-page": "442",
"issue": "7",
"journal-title": "Nat Rev Immunol",
"key": "10.3346/jkms.2022.37.e102_ref32",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1186/s13293-020-00304-9",
"author": "Gebhard",
"doi-asserted-by": "crossref",
"first-page": "29",
"issue": "1",
"journal-title": "Biol Sex Differ",
"key": "10.3346/jkms.2022.37.e102_ref33",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2021.36.e248",
"author": "Moon",
"doi-asserted-by": "crossref",
"first-page": "e248",
"issue": "35",
"journal-title": "J Korean Med Sci",
"key": "10.3346/jkms.2022.37.e102_ref34",
"volume": "36",
"year": "2021"
},
{
"key": "10.3346/jkms.2022.37.e102_ref35",
"unstructured": "Clinical Trials Arena. Celltrion's COVID-19 drug regdanvimab meets Phase III endpoints. Updated 2021. Accessed November 1, 2021. https://www.clinicaltrialsarena.com/news/celltrion-regdanvimab-phaseiii-data/"
},
{
"key": "10.3346/jkms.2022.37.e102_ref36",
"unstructured": "Monoclonal regdanvimab cuts COVID progression risk in placebo trial. IDWeek, September 29-October 3, 2021. Updated 2021. Accessed November 1, 2021. https://www.natap.org/2021/IDWeek/IDWeek_43.htm"
},
{
"first-page": "49",
"issue": "1621",
"journal-title": "Med Lett Drugs Ther",
"key": "10.3346/jkms.2022.37.e102_ref37",
"volume": "63",
"year": "2021"
},
{
"key": "10.3346/jkms.2022.37.e102_ref38",
"unstructured": "FDA. Fact sheet for health care providers. Emergency Use Authorization (EUA) of bamlanivimab and etesevimab. Updated 2021. Accessed November 1, 2021. https://bit.ly/3qfS6DN"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "10.3346/jkms.2022.37.e102_ref39",
"volume": "384",
"year": "2021"
},
{
"first-page": "201",
"issue": "1614",
"journal-title": "Med Lett Drugs Ther",
"key": "10.3346/jkms.2022.37.e102_ref40",
"volume": "62",
"year": "2020"
},
{
"key": "10.3346/jkms.2022.37.e102_ref41",
"unstructured": "FDA. Fact sheet for health care providers. Emergency Use Authorization (EUA) of casirivimab and imdevimab. Updated 2021. Accessed November 1, 2021. http://e-lactancia.org/media/papers/casirivimabimdevimab-DS-FDA-Regeneron2020.pdf"
},
{
"author": "Orders",
"first-page": "97",
"issue": "1627",
"journal-title": "Med Lett Drugs Ther",
"key": "10.3346/jkms.2022.37.e102_ref42",
"volume": "63",
"year": "2021"
},
{
"key": "10.3346/jkms.2022.37.e102_ref43",
"unstructured": "FDA. Fact sheet for health care providers. Emergency Use Authorization (EUA) of sotrovimab. Updated 2021. Accessed November 1, 2021. https://www.fda.gov/media/149534/download"
},
{
"DOI": "10.3390/v13040628",
"author": "Hurt",
"doi-asserted-by": "crossref",
"first-page": "628",
"issue": "4",
"journal-title": "Viruses",
"key": "10.3346/jkms.2022.37.e102_ref44",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1126/science.abd0831",
"author": "Baum",
"doi-asserted-by": "crossref",
"first-page": "1014",
"issue": "6506",
"journal-title": "Science",
"key": "10.3346/jkms.2022.37.e102_ref45",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.bbrc.2021.06.016",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.3346/jkms.2022.37.e102_ref46",
"volume": "566",
"year": "2021"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://jkms.org/DOIx.php?id=10.3346/jkms.2022.37.e102"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Effectiveness and Safety of Regdanvimab in Patients With Mild-To-Moderate COVID-19: A Retrospective Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3346/crossmark_policy",
"volume": "37"
}