Inhaled Sargramostim (Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor) for COVID-19-Associated Acute Hypoxemia: Results of the Phase 2, Randomized, Open-Label Trial (iLeukPulm)
et al., Military Medicine, doi:10.1093/milmed/usac362, iLeukPulm, NCT04411680, Dec 2022
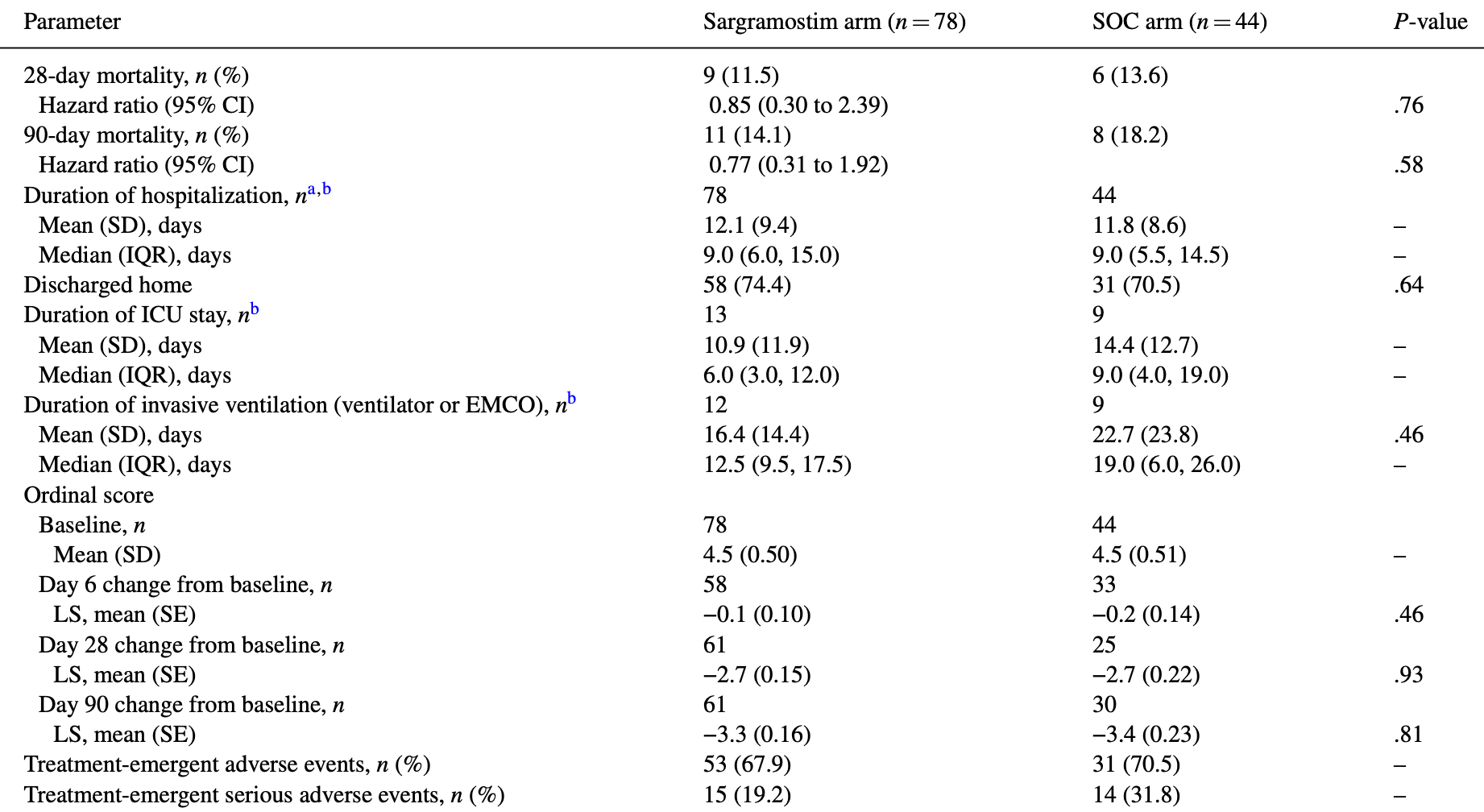
RCT 122 hospitalized COVID-19 patients showing improved oxygenation with inhaled sargramostim (GM-CSF) treatment. There was no significant difference in intubation rate, mortality, or adverse events.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 23.0% lower, HR 0.77, p = 0.58, treatment 78, control 44, day 90.
|
|
risk of death, 15.0% lower, HR 0.85, p = 0.76, treatment 78, control 44, day 28.
|
|
risk of mechanical ventilation, 27.5% lower, RR 0.73, p = 0.58, treatment 9 of 78 (11.5%), control 7 of 44 (15.9%), NNT 23, day 14.
|
|
no improvement in P(A-a)O2, 56.3% lower, RR 0.44, p = 0.04, treatment 10 of 63 (15.9%), control 12 of 33 (36.4%), NNT 4.9.
|
|
hospitalization time, 2.5% higher, relative time 1.03, p = 0.86, treatment mean 12.1 (±9.4) n=78, control mean 11.8 (±8.6) n=44.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Paine et al., 2 Dec 2022, Randomized Controlled Trial, USA, peer-reviewed, mean age 60.4, 14 authors, study period 19 August, 2020 - 17 February, 2021, trial NCT04411680 (history) (iLeukPulm).
Inhaled Sargramostim (Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor) for COVID-19-Associated Acute Hypoxemia: Results of the Phase 2, Randomized, Open-Label Trial (iLeukPulm)
Military Medicine, doi:10.1093/milmed/usac362
Introduction: Granulocyte-macrophage colony-stimulating factor (GM-CSF), a protein produced in the lung, is essential for pulmonary host defense and alveolar integrity. Prior studies suggest potential benefits in several pulmonary conditions, including acute respiratory distress syndrome and viral infections. This trial evaluated the effect of the addition of inhaled sargramostim (yeast-derived, glycosylated recombinant human GM-CSF) to standard of care (SOC) on oxygenation and clinical outcomes in patients with COVID-19-associated acute hypoxemia.
Materials and Methods: A randomized, controlled, open-label trial of hospitalized adults with COVID-19-associated hypoxemia (oxygen saturation <93% on ≥2 L/min oxygen supplementation and/or PaO 2 /FiO 2 <350) randomized 2:1 to inhaled sargramostim (125 mcg twice daily for 5 days) plus SOC versus SOC alone. Institutional SOC before and during the study was not limited. Primary outcomes were change in the alveolar-arterial oxygen gradient (P(A-a)O 2 ) by day 6 and the percentage of patients intubated within 14 days. Safety evaluations included treatment-emergent adverse events. Efficacy analyses were based on the modified intent-to-treat population, the subset of the intent-to-treat population that received ≥1 dose of any study treatment (sargramostim and/or SOC). An analysis of covariance approach was used to analyze changes in oxygenation measures. The intubation rate was analyzed using the chi-squared test. All analyses are considered descriptive. The study was institutional review board approved.
Results: In total, 122 patients were treated (sargramostim, n = 78; SOC, n = 44). The sargramostim arm experienced greater improvement in P(A-a)O 2 by day 6 compared to SOC alone (least squares [LS] mean change from baseline [SE]: -102.3 [19.4] versus -30.5 [26.9] mmHg; LS mean difference: -71.7 [SE 33.2, 95% CI -137.7 to -5.8]; P = .033; n = 96). By day 14, 11.5% (9/78) of sargramostim and 15.9% (7/44) of SOC arms required intubation (P = .49). The 28-day mortality was 11.5% (9/78) and 13.6% (6/44) in the sargramostim and SOC arms, respectively (hazard ratio 0.85; P = .76). Treatment-emergent adverse events occurred in 67.9% (53/78) and 70.5% (31/44) on the sargramostim and SOC arms, respectively.
Conclusions: The addition of inhaled sargramostim to SOC improved P(A-a)O 2 , a measure of oxygenation, by day 6 in hospitalized patients with COVID-19-associated acute hypoxemia and was well tolerated. Inhaled sargramostim is delivered directly
SUPPLEMENTARY MATERIAL Supplementary material is available at Military Medicine online.
CONFLICT OF INTEREST STATEMENT The institutions for R.P., R.C., E. Scott Halstead, J.N., D.
CLINICAL TRIAL REGISTRATION NUMBER The trial was registered with ClinicalTrials.gov (NCT04411680) on May 29, 2020, with E. Scott Halstead, MD, PhD as the principal investigator.
INSTITUTIONAL REVIEW BOARD (HUMAN SUBJECTS) The
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) Not applicable.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
INSTITUTIONAL CLEARANCE Institutional clearance does not apply.
References
Ali, Ali, Iqbal, COVID-19 vaccination: concerns about its accessibility, affordability, and acceptability, Front Med, doi:10.3389/fmed.2021.647294
Arentz, Yim, Klaff, Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state, JAMA, doi:10.1001/jama.2020.4326
Armstrong, Kane, Kursumovic, Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies, Anaesthesia, doi:10.1111/anae.15425
Campo, Mariani, Paracchini, Whole lung lavage followed by inhaled sargramostim as therapy of autoimmune pulmonary alveolar proteinosis, Am J Respir Crit Care Med
Choi, Koch, Wu, Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis, Nat Med, doi:10.1038/s41591-021-01527-y
Criner, Lang, Gottlieb, Anti-granulocyte-macrophage colony-stimulating factor monoclonal antibody gimsilumab for COVID-19 pneumonia: a randomized, double-blind, placebocontrolled trial, Am J Respir Crit Care Med, doi:10.1164/rccm.202108-1859OC
De Roos, Kilsdonk, Hekking, Chest computed tomography and alveolar-arterial oxygen gradient as rapid tools to diagnose and triage mildly symptomatic COVID-19 pneumonia patients, ERJ Open Res, doi:10.1183/23120541.-00737-2020
Hall, Joshi, Leal, Immune immunomodulation in coronavirus disease 2019 (COVID-19): strategic considerations for personalized therapeutic intervention, Clin Infect Dis, doi:10.1093/cid/ciaa904
Hall, Knatz, Vetterly, Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome, Intensive Care Med, doi:10.1007/s00134-010--2088-x
Halstead, Umstead, Davies, GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization, Respir Res, doi:10.1186/s12931-017-0708-5
Harris, Massie, Role of alveolar-arterial gradient in partial pressure of oxygen and PaO 2 /fraction of inspired oxygen ratio measurements in assessment of pulmonary dysfunction, AANA J
Herold, Hoegner, Vadasz, Inhaled granulocyte/macrophage colony-stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.201311-2041LE
Huang, Barnes, Feng, GM-CSF in the lung protects against lethal influenza infection, Am J Respir Crit Care Med, doi:10.1164/rccm.201012-2036OC
Kim, Garg, 'halloran, Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET), Clin Infect Dis, doi:10.1093/cid/ciaa1012
Lee, Miller, Ataya, Opportunistic infection associated with elevated GM-CSF autoantibodies: a case series and review of the literature, Open Forum Infect Dis, doi:10.1093/ofid/ofac146
Mackey, Ayers, Kondo, Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review, Ann Intern Med, doi:10.7326/M20-6306
Mathias, Szpila, Moore, A review of GM-CSF therapy in sepsis, Medicine, doi:10.1097/MD.0000000000002044
Meisel, Schefold, Pschowski, Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial, Am J Respir Crit Care Med, doi:10.1164/rccm.200903-0363OC
Paine, Standiford, Dechert, A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury, Crit Care Med, doi:10.1097/CCM.0b013e31822d7bf0
Rando, Wellhausen, Ghosh, Identification and development of therapeutics for COVID-19, mSystems, doi:10.1128/mSystems.00233-21
Rosik, Lechowicz, An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment, Drug Resist Updat, doi:10.1016/j.drup.2021.100794
Rosler, Herold, Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumoniaa new therapeutic strategy?, Mol Cell Pediatr, doi:10.1186/s40348-016-0055-5
Schneider, Nobs, Kurrer, Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages, Nat Immunol, doi:10.1038/ni.3005
Sever-Chroneos, Murthy, Davis, GM-CSF modulates pulmonary resistance to influenza A infection, Antiviral Res, doi:10.1016/j.antiviral.2011.08.022
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.m2980
Tazawa, Trapnell, Inoue, Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis, Am J Respir Crit Care Med, doi:10.1164/rccm.200906-0978OC
Tazawa, Ueda, Abe, Inhaled GM-CSF for pulmonary alveolar proteinosis, N Engl J Med, doi:10.1056/NEJMoa1816216
Tisoncik, Korth, Simmons, Into the eye of the cytokine storm, Microbiol Mol Biol Rev, doi:10.1128/MMBR.05015-11
Trapnell, Whitsett, Nakata, Pulmonary alveolar proteinosis, N Engl J Med, doi:10.1056/NEJMra023226
Triggle, Bansal, Ding, A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic, Front Immunol, doi:10.3389/fimmu.2021.631139
Tzotzos, Fischer, Fischer, Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey, Crit Care, doi:10.1186/s13054-020-03240-7
Unkel, Hoegner, Clausen, Alveolar epithelial cells orchestrate DC function in murine viral pneumonia, J Clin Invest, doi:10.1172/JCI62139
Wang, Mao, Klein, Diverse functional autoantibodies in patients with COVID-19, Nature, doi:10.1038/s41586-021-03631-y
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wessendarp, Watanabe-Chailland, Liu, Role of GM-CSF in regulating metabolism and mitochondrial functions critical to macrophage proliferation, Mitochondrion, doi:10.1016/j.mito.2021.10.009
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1093/milmed/usac362",
"ISSN": [
"0026-4075",
"1930-613X"
],
"URL": "http://dx.doi.org/10.1093/milmed/usac362",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>Granulocyte-macrophage colony-stimulating factor (GM-CSF), a protein produced in the lung, is essential for pulmonary host defense and alveolar integrity. Prior studies suggest potential benefits in several pulmonary conditions, including acute respiratory distress syndrome and viral infections. This trial evaluated the effect of the addition of inhaled sargramostim (yeast-derived, glycosylated recombinant human GM-CSF) to standard of care (SOC) on oxygenation and clinical outcomes in patients with COVID-19-associated acute hypoxemia.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Materials and Methods</jats:title>\n <jats:p>A randomized, controlled, open-label trial of hospitalized adults with COVID-19-associated hypoxemia (oxygen saturation &lt;93% on ≥2 L/min oxygen supplementation and/or PaO2/FiO2 &lt;350) randomized 2:1 to inhaled sargramostim (125 mcg twice daily for 5 days) plus SOC versus SOC alone. Institutional SOC before and during the study was not limited. Primary outcomes were change in the alveolar–arterial oxygen gradient (P(A–a)O2) by day 6 and the percentage of patients intubated within 14 days. Safety evaluations included treatment-emergent adverse events. Efficacy analyses were based on the modified intent-to-treat population, the subset of the intent-to-treat population that received ≥1 dose of any study treatment (sargramostim and/or SOC). An analysis of covariance approach was used to analyze changes in oxygenation measures. The intubation rate was analyzed using the chi-squared test. All analyses are considered descriptive. The study was institutional review board approved.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>In total, 122 patients were treated (sargramostim, n = 78; SOC, n = 44). The sargramostim arm experienced greater improvement in P(A–a)O2 by day 6 compared to SOC alone (least squares [LS] mean change from baseline [SE]: −102.3 [19.4] versus −30.5 [26.9] mmHg; LS mean difference: −71.7 [SE 33.2, 95% CI −137.7 to −5.8]; P = .033; n = 96). By day 14, 11.5% (9/78) of sargramostim and 15.9% (7/44) of SOC arms required intubation (P = .49). The 28-day mortality was 11.5% (9/78) and 13.6% (6/44) in the sargramostim and SOC arms, respectively (hazard ratio 0.85; P = .76). Treatment-emergent adverse events occurred in 67.9% (53/78) and 70.5% (31/44) on the sargramostim and SOC arms, respectively.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The addition of inhaled sargramostim to SOC improved P(A–a)O2, a measure of oxygenation, by day 6 in hospitalized patients with COVID-19-associated acute hypoxemia and was well tolerated. Inhaled sargramostim is delivered directly to the lung, minimizing systemic effects, and is simple to administer making it a feasible treatment option in patients in settings where other therapy routes may be difficult. Although proportionally lower rates of intubation and mortality were observed in sargramostim-treated patients, this study was insufficiently powered to demonstrate significant changes in these outcomes. However, the significant improvement in gas exchange with sargramostim shows this inhalational treatment enhances pulmonary efficiency in this severe respiratory illness. These data provide strong support for further evaluation of sargramostim in high-risk patients with COVID-19.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Division of Respiratory, Critical Care and Occupational Pulmonary Medicine, University of Utah School of Medicine , Salt Lake City, UT 84132, USA"
}
],
"family": "Paine",
"given": "Robert",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care, TidalHealth Peninsula Regional Medical Center , Salisbury, MD 21801, USA"
}
],
"family": "Chasse",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pediatric Critical Care Medicine, Department of Pediatrics, Penn State University , Hershey, PA 17033, USA"
}
],
"family": "Halstead",
"given": "E Scott",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Critical Care, Richmond University Medical Center , Staten Island, NY 10310, USA"
}
],
"family": "Nfonoyim",
"given": "Jay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology and Oncology, Providence St. Jude Medical Center , Fullerton, CA 92835, USA"
}
],
"family": "Park",
"given": "David J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology and Medical Oncology, Providence St. Joseph Hospital , Orange, CA 92868, USA"
}
],
"family": "Byun",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary, Critical Care and Sleep Medicine, University of Texas Health Science Center , Houston, TX 77030, USA"
}
],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary and Critical Care, Great Plains Health , North Platte, NE 69101, USA"
}
],
"family": "Molina-Pallete",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory, Critical Care and Occupational Pulmonary Medicine, University of Utah School of Medicine , Salt Lake City, UT 84132, USA"
}
],
"family": "Harris",
"given": "Estelle S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Partner Therapeutics, Inc. , Lexington, MA 02421, USA"
}
],
"family": "Garner",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Partner Therapeutics, Inc. , Lexington, MA 02421, USA"
}
],
"family": "Simms",
"given": "Lorinda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Partner Therapeutics, Inc. , Lexington, MA 02421, USA"
}
],
"family": "Ahuja",
"given": "Sanjeev",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Partner Therapeutics, Inc. , Lexington, MA 02421, USA"
}
],
"family": "McManus",
"given": "John L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Partner Therapeutics, Inc. , Lexington, MA 02421, USA"
}
],
"family": "Roychowdhury",
"given": "Debasish F",
"sequence": "additional"
}
],
"container-title": "Military Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
2
]
],
"date-time": "2022-12-02T14:55:23Z",
"timestamp": 1669992923000
},
"deposited": {
"date-parts": [
[
2023,
7,
23
]
],
"date-time": "2023-07-23T01:16:12Z",
"timestamp": 1690074972000
},
"funder": [
{
"award": [
"MCDC2006-012"
],
"name": "Joint Project Manager for Chemical, Biological, Radiological, and Nuclear Medical"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
8
]
],
"date-time": "2024-03-08T10:47:06Z",
"timestamp": 1709894826330
},
"is-referenced-by-count": 5,
"issue": "7-8",
"issued": {
"date-parts": [
[
2022,
12,
2
]
]
},
"journal-issue": {
"issue": "7-8",
"published-online": {
"date-parts": [
[
2022,
12,
2
]
]
},
"published-print": {
"date-parts": [
[
2023,
7,
22
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
2
]
],
"date-time": "2022-12-02T00:00:00Z",
"timestamp": 1669939200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/milmed/article-pdf/188/7-8/e2629/50937134/usac362.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/milmed/article-pdf/188/7-8/e2629/50937134/usac362.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "e2629-e2638",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
12,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
2
]
]
},
"published-other": {
"date-parts": [
[
2023,
7,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
7,
22
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"article-title": "Telltale signs of a ‘Tripledemic’",
"author": "Cornonavirus Resource Center",
"key": "2023072301103648900_R1"
},
{
"DOI": "10.1186/s13054-020-03240-7",
"article-title": "Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey",
"author": "Tzotzos",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Crit Care",
"key": "2023072301103648900_R2",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1111/anae.15425",
"article-title": "Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies",
"author": "Armstrong",
"doi-asserted-by": "publisher",
"first-page": "537",
"issue": "4",
"journal-title": "Anaesthesia",
"key": "2023072301103648900_R3",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.3331",
"article-title": "Four-month clinical status of a cohort of patients after hospitalization for COVID-19",
"author": "The Writing Committee for the COMEBAC Study Group",
"doi-asserted-by": "publisher",
"first-page": "1525",
"issue": "15",
"journal-title": "JAMA",
"key": "2023072301103648900_R4",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "2023072301103648900_R5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "2023072301103648900_R6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1128/mSystems.00233-21",
"article-title": "Identification and development of therapeutics for COVID-19",
"author": "Rando",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "mSystems",
"key": "2023072301103648900_R7",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.631139",
"article-title": "A comprehensive review of viral characteristics, transmission, pathophysiology, immune response, and management of SARS-CoV-2 and COVID-19 as a basis for controlling the pandemic",
"author": "Triggle",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "2023072301103648900_R8",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "2023072301103648900_R9",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.drup.2021.100794",
"article-title": "An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment",
"author": "Drożdżal",
"doi-asserted-by": "publisher",
"journal-title": "Drug Resist Updat",
"key": "2023072301103648900_R10",
"volume": "59",
"year": "2021"
},
{
"article-title": "Emergency use authorization",
"author": "US Food and Drug Administration",
"key": "2023072301103648900_R11"
},
{
"DOI": "10.1038/s41591-021-01527-y",
"article-title": "Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "2025",
"issue": "11",
"journal-title": "Nat Med",
"key": "2023072301103648900_R12",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.647294",
"article-title": "COVID-19 vaccination: concerns about its accessibility, affordability, and acceptability",
"author": "Ali",
"doi-asserted-by": "publisher",
"journal-title": "Front Med (Lausanne)",
"key": "2023072301103648900_R13",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1038/ni.3005",
"article-title": "Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages",
"author": "Schneider",
"doi-asserted-by": "publisher",
"first-page": "1026",
"issue": "11",
"journal-title": "Nat Immunol",
"key": "2023072301103648900_R14",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.1056/NEJMra023226",
"article-title": "Pulmonary alveolar proteinosis",
"author": "Trapnell",
"doi-asserted-by": "publisher",
"first-page": "2527",
"issue": "26",
"journal-title": "N Engl J Med",
"key": "2023072301103648900_R15",
"volume": "349",
"year": "2003"
},
{
"DOI": "10.1016/j.antiviral.2011.08.022",
"article-title": "GM-CSF modulates pulmonary resistance to influenza A infection",
"author": "Sever-Chroneos",
"doi-asserted-by": "publisher",
"first-page": "319",
"issue": "2",
"journal-title": "Antiviral Res",
"key": "2023072301103648900_R16",
"volume": "92",
"year": "2011"
},
{
"DOI": "10.1016/j.mito.2021.10.009",
"article-title": "Role of GM-CSF in regulating metabolism and mitochondrial functions critical to macrophage proliferation",
"author": "Wessendarp",
"doi-asserted-by": "publisher",
"first-page": "85",
"journal-title": "Mitochondrion",
"key": "2023072301103648900_R17",
"volume": "62",
"year": "2022"
},
{
"DOI": "10.1172/JCI62139",
"article-title": "Alveolar epithelial cells orchestrate DC function in murine viral pneumonia",
"author": "Unkel",
"doi-asserted-by": "publisher",
"first-page": "3652",
"issue": "10",
"journal-title": "J Clin Invest",
"key": "2023072301103648900_R18",
"volume": "122",
"year": "2012"
},
{
"DOI": "10.1093/ofid/ofac146",
"article-title": "Opportunistic infection associated with elevated GM-CSF autoantibodies: a case series and review of the literature",
"author": "Lee",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "Open Forum Infect Dis",
"key": "2023072301103648900_R19",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03631-y",
"article-title": "Diverse functional autoantibodies in patients with COVID-19",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "283",
"issue": "7866",
"journal-title": "Nature",
"key": "2023072301103648900_R20",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1186/s12931-017-0708-5",
"article-title": "GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization",
"author": "Halstead",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Respir Res",
"key": "2023072301103648900_R21",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1186/s40348-016-0055-5",
"article-title": "Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia—a new therapeutic strategy?",
"author": "Rosler",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Mol Cell Pediatr",
"key": "2023072301103648900_R22",
"volume": "3",
"year": "2016"
},
{
"DOI": "10.1164/rccm.201311-2041LE",
"article-title": "Inhaled granulocyte/macrophage colony-stimulating factor as treatment of pneumonia-associated acute respiratory distress syndrome",
"author": "Herold",
"doi-asserted-by": "publisher",
"first-page": "609",
"issue": "5",
"journal-title": "Am J Respir Crit Care Med",
"key": "2023072301103648900_R23",
"volume": "189",
"year": "2014"
},
{
"DOI": "10.1056/NEJMoa1816216",
"article-title": "Inhaled GM-CSF for pulmonary alveolar proteinosis",
"author": "Tazawa",
"doi-asserted-by": "publisher",
"first-page": "923",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "2023072301103648900_R24",
"volume": "381",
"year": "2019"
},
{
"DOI": "10.1164/rccm.200906-0978OC",
"article-title": "Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis",
"author": "Tazawa",
"doi-asserted-by": "publisher",
"first-page": "1345",
"issue": "12",
"journal-title": "Am J Respir Crit Care Med",
"key": "2023072301103648900_R25",
"volume": "181",
"year": "2010"
},
{
"article-title": "Whole lung lavage followed by inhaled sargramostim as therapy of autoimmune pulmonary alveolar proteinosis",
"author": "Campo",
"journal-title": "Am J Respir Crit Care Med",
"key": "2023072301103648900_R26",
"volume": "193",
"year": "2016"
},
{
"DOI": "10.1097/CCM.0b013e31822d7bf0",
"article-title": "A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury",
"author": "Paine",
"doi-asserted-by": "publisher",
"first-page": "90",
"issue": "1",
"journal-title": "Crit Care Med",
"key": "2023072301103648900_R27",
"volume": "40",
"year": "2012"
},
{
"DOI": "10.1097/MD.0000000000002044",
"article-title": "A review of GM-CSF therapy in sepsis",
"author": "Mathias",
"doi-asserted-by": "publisher",
"issue": "50",
"journal-title": "Medicine (Baltimore)",
"key": "2023072301103648900_R28",
"volume": "94",
"year": "2015"
},
{
"DOI": "10.1164/rccm.200903-0363OC",
"article-title": "Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial",
"author": "Meisel",
"doi-asserted-by": "publisher",
"first-page": "640",
"issue": "7",
"journal-title": "Am J Respir Crit Care Med",
"key": "2023072301103648900_R29",
"volume": "180",
"year": "2009"
},
{
"DOI": "10.1007/s00134-010-2088-x",
"article-title": "Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome",
"author": "Hall",
"doi-asserted-by": "publisher",
"first-page": "525",
"issue": "3",
"journal-title": "Intensive Care Med",
"key": "2023072301103648900_R30",
"volume": "37",
"year": "2011"
},
{
"article-title": "LEUKINE® (sargramostim) for injection [Prescribing Information]",
"key": "2023072301103648900_R31",
"year": "2022"
},
{
"DOI": "10.7326/M20-6306",
"article-title": "Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review",
"author": "Mackey",
"doi-asserted-by": "publisher",
"first-page": "362",
"issue": "3",
"journal-title": "Ann Intern Med",
"key": "2023072301103648900_R32",
"volume": "174",
"year": "2021"
},
{
"article-title": "Collection of race and ethnicity data in clinical trials",
"author": "Guidance for Industry and Food and Drug Administration Staff",
"key": "2023072301103648900_R33"
},
{
"DOI": "10.1183/23120541.00737-2020",
"article-title": "Chest computed tomography and alveolar-arterial oxygen gradient as rapid tools to diagnose and triage mildly symptomatic COVID-19 pneumonia patients",
"author": "de Roos",
"doi-asserted-by": "publisher",
"first-page": "00737",
"issue": "1",
"journal-title": "ERJ Open Res",
"key": "2023072301103648900_R34",
"volume": "7",
"year": "2021"
},
{
"article-title": "Role of alveolar-arterial gradient in partial pressure of oxygen and PaO2/fraction of inspired oxygen ratio measurements in assessment of pulmonary dysfunction",
"author": "Harris",
"first-page": "214",
"issue": "3",
"journal-title": "AANA J",
"key": "2023072301103648900_R35",
"volume": "87",
"year": "2019"
},
{
"DOI": "10.1001/jama.2020.4326",
"article-title": "Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state",
"author": "Arentz",
"doi-asserted-by": "publisher",
"first-page": "1612",
"issue": "16",
"journal-title": "JAMA",
"key": "2023072301103648900_R36",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "2023072301103648900_R37",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1012",
"article-title": "Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET)",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "e206",
"issue": "9",
"journal-title": "Clin Infect Dis",
"key": "2023072301103648900_R38",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa904",
"article-title": "Immune immunomodulation in coronavirus disease 2019 (COVID-19): strategic considerations for personalized therapeutic intervention",
"author": "Hall",
"doi-asserted-by": "publisher",
"first-page": "144",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "2023072301103648900_R39",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1128/MMBR.05015-11",
"article-title": "Into the eye of the cytokine storm",
"author": "Tisoncik",
"doi-asserted-by": "publisher",
"first-page": "16",
"issue": "1",
"journal-title": "Microbiol Mol Biol Rev",
"key": "2023072301103648900_R40",
"volume": "76",
"year": "2012"
},
{
"DOI": "10.1164/rccm.201012-2036OC",
"article-title": "GM-CSF in the lung protects against lethal influenza infection",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "259",
"issue": "2",
"journal-title": "Am J Respir Crit Care Med",
"key": "2023072301103648900_R41",
"volume": "184",
"year": "2011"
},
{
"DOI": "10.1164/rccm.202108-1859OC",
"article-title": "Anti-granulocyte-macrophage colony-stimulating factor monoclonal antibody gimsilumab for COVID-19 pneumonia: a randomized, double-blind, placebo-controlled trial",
"author": "Criner",
"doi-asserted-by": "publisher",
"first-page": "1290",
"issue": "11",
"journal-title": "Am J Respir Crit Care Med",
"key": "2023072301103648900_R42",
"volume": "205",
"year": "2022"
}
],
"reference-count": 42,
"references-count": 42,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/milmed/article/188/7-8/e2629/6865122"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Inhaled Sargramostim (Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor) for COVID-19-Associated Acute Hypoxemia: Results of the Phase 2, Randomized, Open-Label Trial (iLeukPulm)",
"type": "journal-article",
"volume": "188"
}