Seroconversion and outcomes after initial and booster COVID‐19 vaccination in adults with hematologic malignancies
et al., Cancer, doi:10.1002/cncr.34354, Jul 2022
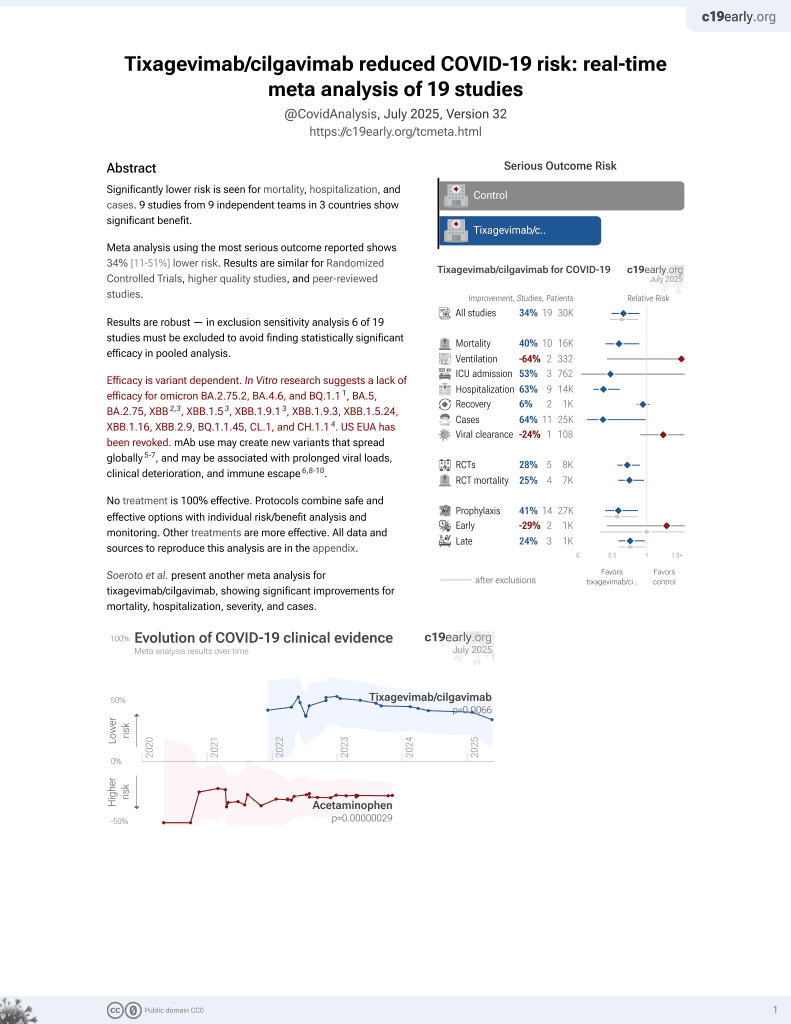
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
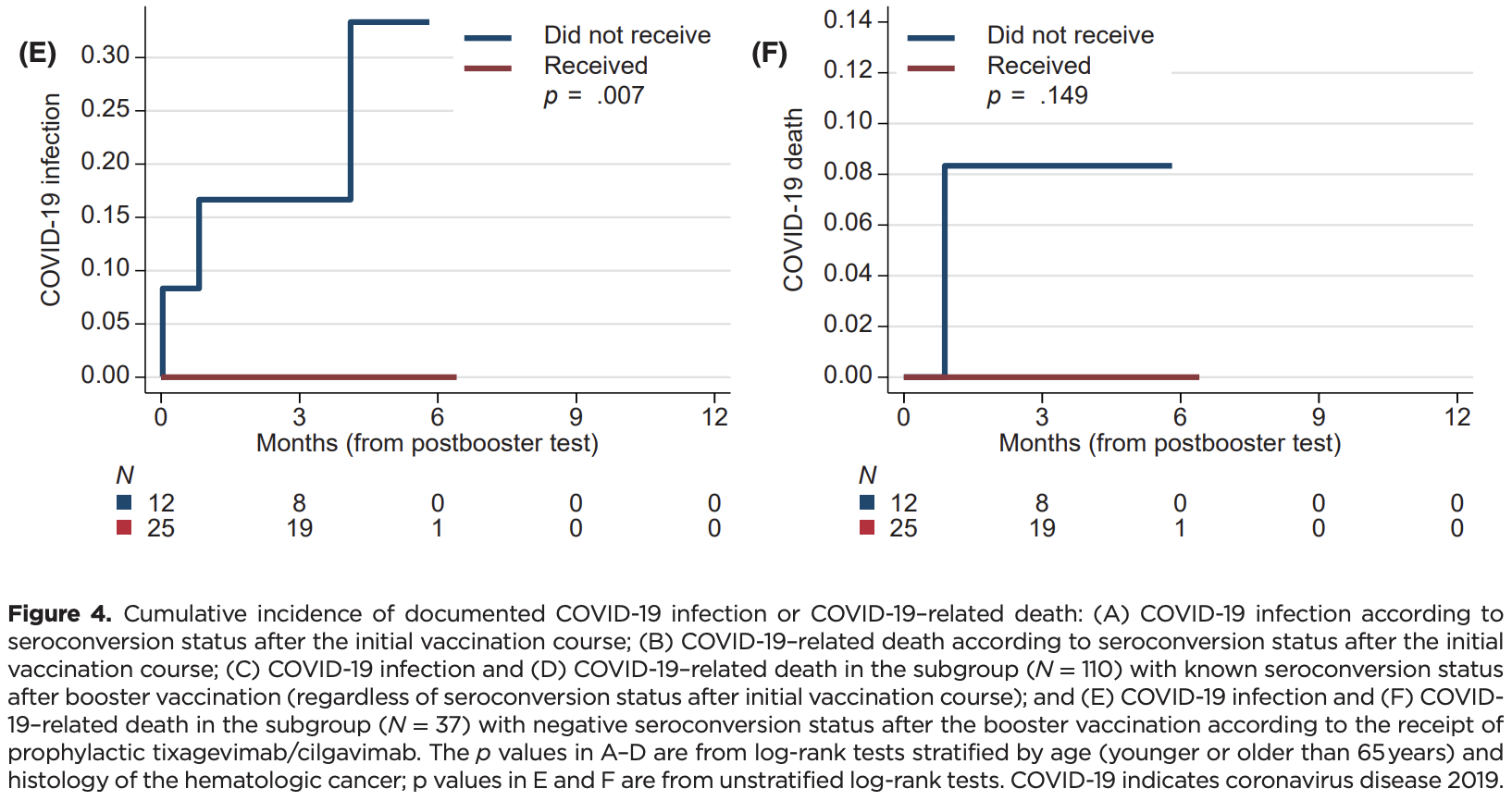
Retrospective 378 patients with hematologic malignancies analyzing seroconversion and outcomes post-vaccination. Among 25 seronegative patients after booster vaccination who received tixagevimab/cilgavimab prophylaxis, no COVID-19 infections occurred, whereas 3 infections and 1 death occurred among 12 comparable patients not receiving prophylaxis.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments5.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 75.5% lower, RR 0.24, p = 0.32, treatment 0 of 25 (0.0%), control 1 of 12 (8.3%), NNT 12, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of case, 90.2% lower, RR 0.10, p = 0.03, treatment 0 of 25 (0.0%), control 3 of 12 (25.0%), NNT 4.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
3.
Uraki et al., Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate, iScience, doi:10.1016/j.isci.2023.108147.
Ollila et al., 11 Jul 2022, retrospective, USA, peer-reviewed, 13 authors, study period February 2021 - February 2022.
Contact: thomas_ollila@brown.edu.
Seroconversion and outcomes after initial and booster COVID‐19 vaccination in adults with hematologic malignancies
Cancer, doi:10.1002/cncr.34354
BACKGROUND: Patients with hematologic malignancies have impaired humoral immunity secondary to their malignancy and its treatment, placing them at risk of severe coronavirus disease-19 (COVID-19) infection and reduced response to vaccination. METHODS: The authors retrospectively analyzed serologic responses to initial and booster COVID-19 vaccination in 378 patients with hematologic malignancy and subsequently tracked COVID-19-related outcomes. RESULTS: Seroconversion occurred in 181 patients (48%) after initial vaccination; patients who had active malignancy or those who were recently treated with a B-cell-depleting monoclonal antibody had the lowest rates of seroconversion. For initial nonresponders to vaccination, seroconversion after a booster dose occurred in 48 of 85 patients (56%). The seroconversion rate after the booster was similar for patients on (53%) and off (58%) active therapy (p = .82). Thirty-three patients (8.8%) developed a COVID-19 infection, and there were three COVID-19-related deaths (0.8%). Although no significant association was observed between postvaccination seroconversion and the incidence of COVID-19 infection, no patient with seroconversion died from COVID-19, and no patient who received tixagevimab/cilgavimab (N = 25) was diagnosed with a COVID-19 infection. CONCLUSIONS: Booster vaccinations can promote seroconversion in a significant proportion of patients who are seronegative after the initial vaccination course regardless of the specific vaccine or on/off treatment status at the time of revaccination. Although postvaccination seroconversion may not be associated with a decrease in any (including asymptomatic) COVID-19 infection, the authors' experience suggested that effective vaccination (including a booster), supplemented by passive immunization using tixagevimab/cilgavimab in case of lack of seroconversion, effectively eliminated the risk of COVID-19 death in the otherwise high-risk population.
CONFLICT OF INTEREST Thomas A. Ollila reports a grant from the Rhode Island Foundation outside the submitted work. Peter Barth reports personal fees from Celgene and advisory board service at AbbVie, Janssen, and Sanofi-Aventis outside the submitted work. Adam J. Olszewski reports research funding from Genentech, TG Therapeutics, Celldex Pharmaceuticals, and Precision Bio; and grants from Acrotech Pharma, Adaptive Biotechnologies outside the submitted work. The remaining authors made no disclosures.
References
Abramson, Ghosh, Smith, ADCs, BiTEs, CARs, and small molecules: a new era of targeted therapy in non-Hodgkin lymphoma, ASCO Educ Book, doi:10.1200/edbk_279043
Anderson, Rouphael, Widge, Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults, N Engl J Med, doi:10.1056/NEJMoa2028436
Atmar, Lyke, Deming, Homologous and heterologous covid-19 booster vaccinations, N Engl J Med, doi:10.1056/NEJMoa2116414
Baden, Sahly, Essink, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389
Bange, Han, Wileyto, CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer, Nat Med, doi:10.1038/s41591-021-01386-7
Bedognetti, Zoppoli, Massucco, Impaired response to influenza vaccine associated with persistent memory B cell depletion in non-Hodgkin's lymphoma patients treated with rituximabcontaining regimens, J Immunol, doi:10.4049/jimmunol.1004095
Branagan, Duffy, Gan, Tandem high-dose influenza vaccination is associated with more durable serologic immunity in patients with plasma cell dyscrasias, Blood Adv, doi:10.1182/bloodadvances.2020003880
Chung, Shah, Devlin, Disease-and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies, Blood Cancer Discov, doi:10.1158/2643-3230.Bcd-21-0139
Dahiya, Luetkens, Lutfi, Impaired immune response to COVID-19 vaccination in patients with B-cell malignancies after CD19 CAR T-cell therapy, Blood Adv, doi:10.1182/bloodadvances.2021006112
Gilbert, Montefiori, Mcdermott, Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial, Science, doi:10.1126/science.abm3425
Greenberger, Saltzman, Senefeld, Johnson, Degennaro et al., Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies, Cancer Cell, doi:10.1016/j.ccell.2021.07.012
Gurion, Rozovski, Itchaki, Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies, Haematologica, doi:10.3324/haematol.2021.279216
Herishanu, Rahav, Levi, Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination, Blood, doi:10.1182/blood.2021014085
Hueso, Pouderoux, Pere, Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19, Blood, doi:10.1182/blood.2020008423
Ollila, Butera, Egan, Vincristine sulfate liposome injection with bendamustine and rituximab as first-line therapy for B-cell lymphomas: a phase I study, Oncologist, doi:10.1093/oncolo/oyab079
Ollila, Lu, Masel, Antibody response to COVID-19 vaccination in adults with hematologic malignant disease, JAMA Oncol, doi:10.1001/jamaoncol.2021.4381
Paiva, Grisson, Chan, Validation and performance comparison of three SARS-CoV-2 antibody assays, J Med Virol, doi:10.1002/jmv.26341
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Ribas, Dhodapkar, Campbell, How to provide the needed protection from COVID-19 to patients with hematologic malignancies, Blood Cancer Discov, doi:10.1158/2643-3230.Bcd-21-0166
Ribas, Sengupta, Locke, Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited, Cancer Discov, doi:10.1158/2159-8290.Cd-20-1817
Sadoff, Gars, Shukarev, Interim results of a phase 1-2a trial of Ad26.COV2.S Covid-19 vaccine, N Engl J Med, doi:10.1056/NEJMoa2034201
Sadoff, Gray, Vandebosch, Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19, N Engl J Med, doi:10.1056/NEJMoa2101544
Saini, Tagliamento, Lambertini, Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies, Eur J Cancer
Schmidt, Labaki, Hsu, COVID-19 vaccination and breakthrough infections in patients with cancer, Ann Oncol, doi:10.1016/j.annonc.2021.12.006
Sidler, Born, Schietzel, Trajectories of humoraland cellular immunity and responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy, RMD Open, doi:10.1136/rmdopen-2021-002166
Teh, Coussement, Neoh, Immunogenicity of COVID-19 vaccines in patients with hematological malignancy: a systematic review and meta-analysis, Blood Adv, doi:10.1182/bloodadvances.2021006333
Walensky, Md, Mph, on Signing the Advisory Committee on Immunization Practices' Recommendation for an Additional Dose of an mRNA COVID-19 Vaccine in Moderately Cancer September 15, 2022 to Severely Immunocompromised People
DOI record:
{
"DOI": "10.1002/cncr.34354",
"ISSN": [
"0008-543X",
"1097-0142"
],
"URL": "http://dx.doi.org/10.1002/cncr.34354",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Patients with hematologic malignancies have impaired humoral immunity secondary to their malignancy and its treatment, placing them at risk of severe coronavirus disease‐19 (COVID‐19) infection and reduced response to vaccination.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>The authors retrospectively analyzed serologic responses to initial and booster COVID‐19 vaccination in 378 patients with hematologic malignancy and subsequently tracked COVID‐19–related outcomes.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Seroconversion occurred in 181 patients (48%) after initial vaccination; patients who had active malignancy or those who were recently treated with a B‐cell–depleting monoclonal antibody had the lowest rates of seroconversion. For initial nonresponders to vaccination, seroconversion after a booster dose occurred in 48 of 85 patients (56%). The seroconversion rate after the booster was similar for patients on (53%) and off (58%) active therapy (<jats:italic>p</jats:italic> = .82). Thirty‐three patients (8.8%) developed a COVID‐19 infection, and there were three COVID‐19–related deaths (0.8%). Although no significant association was observed between postvaccination seroconversion and the incidence of COVID‐19 infection, no patient with seroconversion died from COVID‐19, and no patient who received tixagevimab/cilgavimab (<jats:italic>N</jats:italic> = 25) was diagnosed with a COVID‐19 infection.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Booster vaccinations can promote seroconversion in a significant proportion of patients who are seronegative after the initial vaccination course regardless of the specific vaccine or on/off treatment status at the time of revaccination. Although postvaccination seroconversion may not be associated with a decrease in any (including asymptomatic) COVID‐19 infection, the authors' experience suggested that effective vaccination (including a booster), supplemented by passive immunization using tixagevimab/cilgavimab in case of lack of seroconversion, effectively eliminated the risk of COVID‐19 death in the otherwise high‐risk population.</jats:p></jats:sec><jats:sec><jats:title>Lay summary</jats:title><jats:p>\n<jats:list list-type=\"bullet\">\n\n<jats:list-item><jats:p>Patients with hematologic malignancy, especially lymphoma, have an impaired response to coronavirus disease 2019 (COVID‐19) vaccination.</jats:p></jats:list-item>\n\n<jats:list-item><jats:p>In this single‐institution review, less than one half of the patients studied made detectable antibodies.</jats:p></jats:list-item>\n\n<jats:list-item><jats:p>For those who did not make detectable antibodies after initial vaccination, over one half (65%) were able to produce antibodies after booster vaccination.</jats:p></jats:list-item>\n\n<jats:list-item><jats:p>By the end of February 2022, 33 of the original 378 patients had a documented COVID‐19 infection.</jats:p></jats:list-item>\n\n<jats:list-item><jats:p>The only deaths from COVID‐19 were in those who had undetectable antibodies, and no patient who received prophylactic antibody therapy developed a COVID‐19 infection.</jats:p></jats:list-item>\n</jats:list>\n</jats:p></jats:sec>",
"alternative-id": [
"10.1002/cncr.34354"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-03-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-05-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-07-11"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0102-6491",
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"authenticated-orcid": false,
"family": "Ollila",
"given": "Thomas A.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
}
],
"family": "Masel",
"given": "Rebecca H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Reagan",
"given": "John L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Department of Pathology and Laboratory Medicine Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Lu",
"given": "Shaolei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Infectious Disease Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Rogers",
"given": "Ralph D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Paiva",
"given": "Kimberly J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Taher",
"given": "Rashida",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Warren Alpert Medical School of Brown University Providence Rhode Island USA"
}
],
"family": "Burguera‐Couce",
"given": "Ella",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7591-2069",
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"authenticated-orcid": false,
"family": "Zayac",
"given": "Adam S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Yakirevich",
"given": "Inna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"family": "Niroula",
"given": "Rabin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2216-8376",
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"authenticated-orcid": false,
"family": "Barth",
"given": "Peter",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6472-6658",
"affiliation": [
{
"name": "Department of Medicine Alpert Medical School of Brown University Providence Rhode Island USA"
},
{
"name": "Division of Hematology‐Oncology Rhode Island Hospital Providence Rhode Island USA"
}
],
"authenticated-orcid": false,
"family": "Olszewski",
"given": "Adam J.",
"sequence": "additional"
}
],
"container-title": "Cancer",
"container-title-short": "Cancer",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"acsjournals.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T07:01:12Z",
"timestamp": 1657522872000
},
"deposited": {
"date-parts": [
[
2023,
8,
23
]
],
"date-time": "2023-08-23T04:38:02Z",
"timestamp": 1692765482000
},
"indexed": {
"date-parts": [
[
2024,
1,
5
]
],
"date-time": "2024-01-05T12:59:49Z",
"timestamp": 1704459589958
},
"is-referenced-by-count": 24,
"issue": "18",
"issued": {
"date-parts": [
[
2022,
7,
11
]
]
},
"journal-issue": {
"issue": "18",
"published-print": {
"date-parts": [
[
2022,
9,
15
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
11
]
],
"date-time": "2022-07-11T00:00:00Z",
"timestamp": 1657497600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cncr.34354",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/cncr.34354",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cncr.34354",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "3319-3329",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
7,
11
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
11
]
]
},
"published-print": {
"date-parts": [
[
2022,
9,
15
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.ejca.2020.08.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_2_1"
},
{
"DOI": "10.1016/j.annonc.2021.12.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_3_1"
},
{
"DOI": "10.1001/jamaoncol.2021.4381",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_4_1"
},
{
"DOI": "10.1158/2643‐3230.Bcd‐21‐0166",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_5_1"
},
{
"DOI": "10.4049/jimmunol.1004095",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_6_1"
},
{
"DOI": "10.1182/bloodadvances.2021006333",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_7_1"
},
{
"DOI": "10.1182/blood.2020008423",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_8_1"
},
{
"DOI": "10.1056/NEJMoa2035389",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_9_1"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_10_1"
},
{
"DOI": "10.1056/NEJMoa2101544",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_11_1"
},
{
"DOI": "10.1158/2159‐8290.Cd‐20‐1817",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_12_1"
},
{
"key": "e_1_2_7_13_1",
"unstructured": "Centers for Disease Control and Prevention (CDC). Media Statement from CDC Director Rochelle P. Walensky MD MPH on Signing the Advisory Committee on Immunization Practices' Recommendation for an Additional Dose of an mRNA COVID‐19 Vaccine in Moderately to Severely Immunocompromised People. Accessed December 9 2021.https://www.cdc.gov/media/releases/2021/s0813‐additional‐mRNA‐mrna‐dose.html"
},
{
"DOI": "10.1056/NEJMoa2116414",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_14_1"
},
{
"key": "e_1_2_7_15_1",
"unstructured": "Centers for Disease Control and Prevention (CDC). COVID‐19 Vaccines for Moderately or Severely Immunocompromised People. Accessed December 10 2021.https://www.cdc.gov/coronavirus/2019‐ncov/vaccines/recommendations/immuno.html"
},
{
"DOI": "10.3324/haematol.2021.279216",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_16_1"
},
{
"DOI": "10.1002/jmv.26341",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_17_1"
},
{
"DOI": "10.1056/NEJMoa2028436",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_18_1"
},
{
"DOI": "10.1056/NEJMoa2034201",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_19_1"
},
{
"DOI": "10.1158/2643‐3230.Bcd‐21‐0139",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_20_1"
},
{
"DOI": "10.1182/blood.2021014085",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_21_1"
},
{
"DOI": "10.1200/edbk_279043",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_22_1"
},
{
"DOI": "10.1182/bloodadvances.2021006112",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_23_1"
},
{
"DOI": "10.1016/j.ccell.2021.07.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_24_1"
},
{
"DOI": "10.1136/rmdopen‐2021‐002166",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_25_1"
},
{
"DOI": "10.1182/bloodadvances.2020003880",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_26_1"
},
{
"key": "e_1_2_7_27_1",
"unstructured": "World Health Organization (WHO). WHO Global Consultation—What evidence do we have that omicron is evading immunity and what are the implications? Accessed December 20 2021.https://www.who.int/news‐room/events/detail/2021/12/15/default‐calendar/who‐global‐consultation—what‐evidence‐do‐we‐have‐that‐omicron‐is‐evading‐immunity‐and‐what‐are‐the‐implications"
},
{
"DOI": "10.1001/jama.2021.24931",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_28_1"
},
{
"DOI": "10.1126/science.abm3425",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_29_1"
},
{
"DOI": "10.1038/s41591‐021‐01386‐7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_30_1"
},
{
"DOI": "10.1093/oncolo/oyab079",
"doi-asserted-by": "crossref",
"key": "e_1_2_7_31_1",
"unstructured": "OllilaT ButeraJ EganP et al.Vincristine sulfate liposome injection with bendamustine and rituximab as first‐line therapy for B‐cell lymphomas: a phase I study.Oncologist. Published online March 7 2022doi:10.1093/oncolo/oyab079"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://acsjournals.onlinelibrary.wiley.com/doi/10.1002/cncr.34354"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cancer Research",
"Oncology"
],
"subtitle": [],
"title": "Seroconversion and outcomes after initial and booster <scp>COVID</scp>‐19 vaccination in adults with hematologic malignancies",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "128"
}