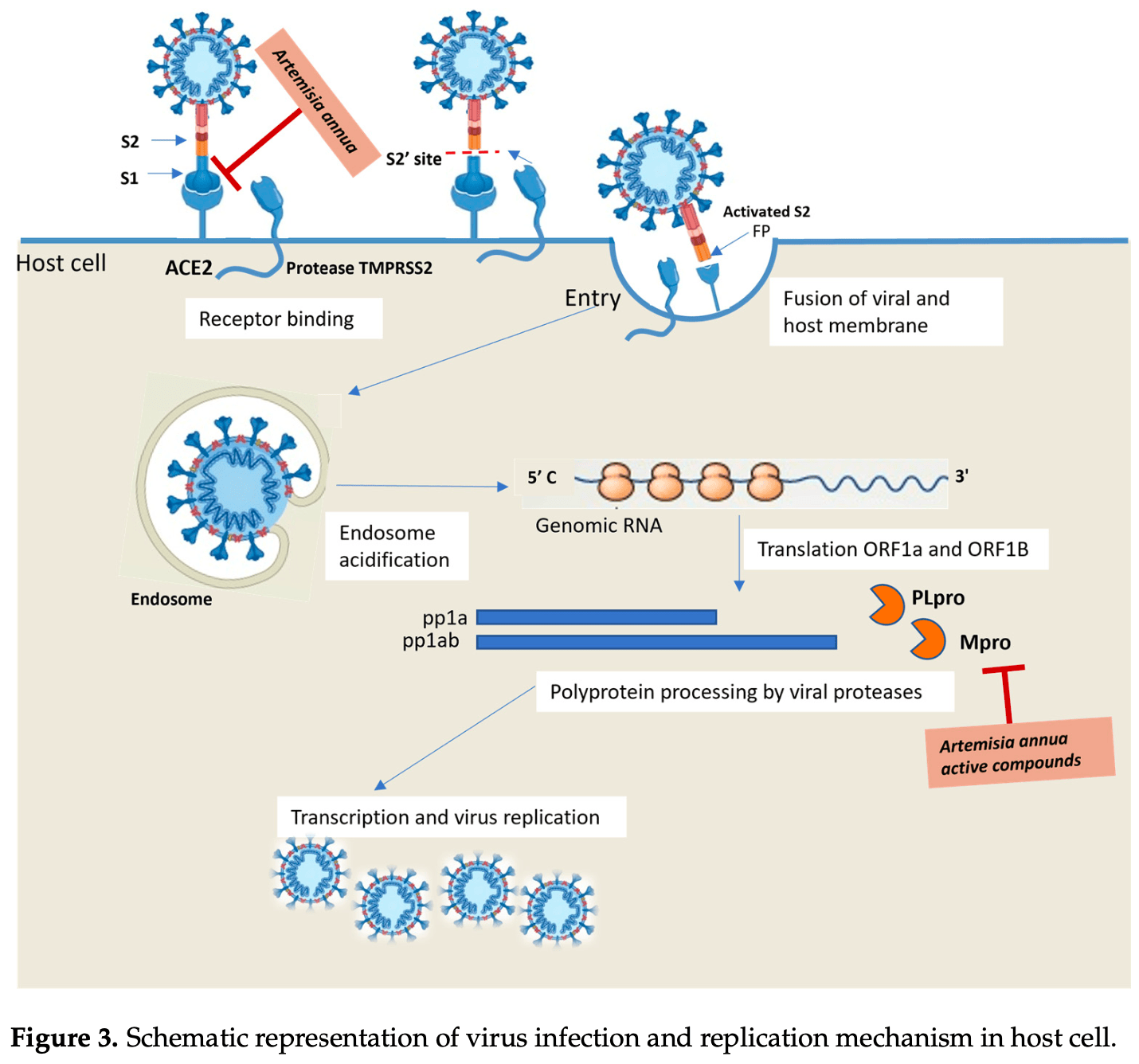
Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment
et al., Molecules, doi:10.3390/molecules29225403, Nov 2024
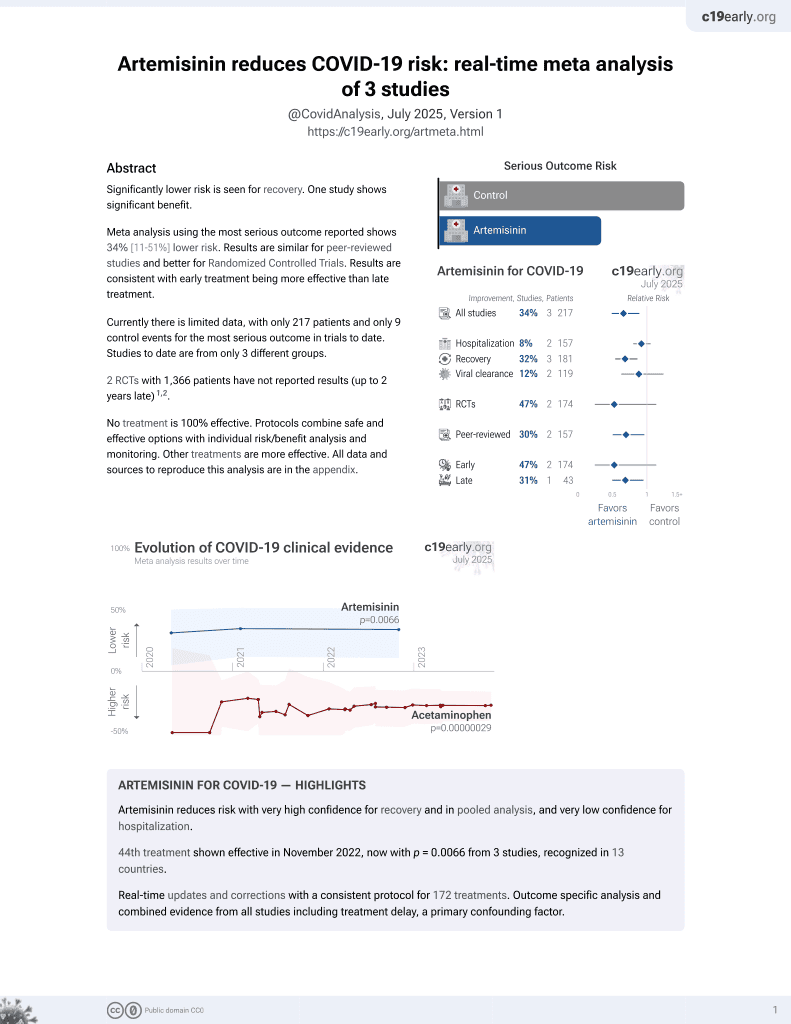
46th treatment shown to reduce risk in
November 2022, now with p = 0.0066 from 3 studies, recognized in 13 countries.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of recent advances in small-molecule hybrid compounds as potential antiviral agents against SARS-CoV-2. Authors provide an overview of hybrids, which are formed by covalently linking two or more pharmacophores, as a promising approach to develop COVID-19 treatments. The review covers artemisinin-based hybrids, peptidomimetic protease inhibitor hybrids, 1,2,3-triazole-based hybrids, thiazole/coumarin-based hybrids, and other miscellaneous hybrids. In vitro studies showed several hybrids had potent antiviral activity against SARS-CoV-2, in some cases comparable to or better than reference drugs, by inhibiting viral targets such as the main protease, RNA-dependent RNA polymerase, and spike protein.
Navacchia et al., 15 Nov 2024, peer-reviewed, 4 authors.
Contact: marialuisa.navacchia@isof.cnr.it (corresponding author), caterina.cinti@cnr.it, mrclne@unife.it, prd@unife.it.
Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment
Molecules, doi:10.3390/molecules29225403
The advantages of a treatment modality that combines two or more therapeutic agents with different mechanisms of action encourage the study of hybrid functional compounds for pharmacological applications. Molecular hybridization, resulting from a covalent combination of two or more pharmacophore units, has emerged as a promising approach to overcome several issues and has also been explored for the design of new drugs for COVID-19 treatment. In this review, we presented an overview of small-molecule hybrids from both natural products and synthetic sources reported in the literature to date with potential antiviral anti-SARS-CoV-2 activity.
References
Abdallah, Abdel-Latif, Elgemeie, Novel Fluorescent Benzothiazolyl-Coumarin Hybrids as Anti-SARS-COVID-2 Agents Supported by Molecular Docking Studies: Design, Synthesis, X-Ray Crystal Structures, DFT, and TD-DFT/PCM Calculations, ACS Omega, doi:10.1021/acsomega.3c01085
Abdel-Rahman, Abdel-Aziz, Canzoneri, Gary, Piazza, Novel Quinazolin-4(3H)-one/Schiff Base Hybrids as Antiproliferative and Phosphodiesterase 4 Inhibitors: Design, Synthesis, and Docking Studies, Arch. Pharm, doi:10.1002/ardp.201400083
Afzal, Kumar, Haider, Ali, Kumar et al., A Review on Anticancer Potential of Bioactive Heterocycle Quinoline, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2014.07.044
Aggarwal, Sumran, An Insight on Medicinal Attributes of 1,2,4-Triazoles, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2020.112652
Ahmad, Abbasi, Shahid, Gul, Abbasi, Molecular Docking, Simulation and MM-PBSA Studies of Nigella Sativa Compounds: A Computational Quest to Identify Potential Natural Antiviral for COVID-19 Treatment, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1775129
Al-Humaidi, Shaaban, Rezki, Aouad, Zakaria et al., 1,2,3-Triazole-Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants, Life, doi:10.3390/life12091341
Aljuhani, Alsehli, Seleem, Alraqa, Ahmed et al., Exploring of N-Phthalimide-Linked 1,2,3-Triazole Analogues with Promising anti-SARS-CoV-2 Activity: Synthesis, Biological Screening, and Molecular Modelling Studies, J. Enzyme Inhib. Med. Chem, doi:10.1080/14756366.2024.2351861
Arasappan, Bennett, Bogen, Venkatraman, Blackman et al., Discovery of Narlaprevir (SCH 900518): A Potent, Second Generation HCV NS3 Serine Protease Inhibitor, ACS Med. Chem. Lett, doi:10.1021/ml9000276
Asiri, Alsayari, Muhsinah, Mabkhot, Hassan, Benzothiazoles as Potential Antiviral Agents, J. Pharm. Pharmacol, doi:10.1111/jphp.13331
Barhoumi, Alghanem, Shaibah, Mansour, Alamri et al., SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril), Front. Immunol, doi:10.3389/fimmu.2021.728896
Bian, Gao, Zhang, He, Mao et al., Effects of SARS-CoV-2 Variants on Vaccine Efficacy and Response Strategies, Expert Rev. Vaccines, doi:10.1080/14760584.2021.1903879
Bonhomme, Poirier, Early Signaling Pathways in Virus-Infected Cells, Curr. Opin. Virol, doi:10.1016/j.coviro.2024.101411
Bravo, Alonso-Moreno, Posadas, Albaladejo, Carrillo-Hermosilla et al., Phenyl-Guanidine Derivatives as Potential Therapeutic Agents for Glioblastoma Multiforme: Catalytic Syntheses, Cytotoxic Effects and DNA Affinity, RSC Adv, doi:10.1039/C5RA17920C
Brevini, Maes, Webb, John, Fuchs et al., FXR Inhibition May Protect from SARS-CoV-2 Infection by Reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Bzówka, Mitusi Ńska, Raczy Ńska, Samol, Tuszy Ński et al., Structural and Evolutionary Analysis Indicate That the SARS-CoV-2 Mpro Is a Challenging Target for Small-Molecule Inhibitor Design, Int. J. Mol. Sci, doi:10.3390/ijms21093099
Cao, Hu, Li, Wang, Xu et al., Anti-SARS-CoV-2 Potential of Artemisinins In Vitro, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00522
Cha, Park, Kim, Angiotensin-(1-9) Ameliorates Pulmonary Arterial Hypertension via Angiotensin Type II Receptor, Korean J. Physiol. Pharmacol, doi:10.4196/kjpp.2018.22.4.447
Chandra, Singh, Mahato, Patel, Fluorine-a Small Magic Bullet Atom in the Drug Development: Perspective to FDA Approved and COVID-19 Recommended Drugs, Chem. Pap, doi:10.1007/s11696-023-02804-5
Chaurasyia, Chawla, Monga, Singh, Rhodanine Derivatives: An Insight into the Synthetic and Medicinal Perspectives as Antimicrobial and Antiviral Agents, Chem. Biol. Drug Des, doi:10.1111/cbdd.14163
Chidambaram, Ali, Alarifi, Radhakrishnan, Akbar, In Silico Molecular Docking: Evaluation of Coumarin Based Derivatives against SARS-CoV-2, J. Infect. Public Health, doi:10.1016/j.jiph.2020.09.002
Citarella, Dimasi, Moi, Passarella, Scala et al., Recent Advances in SARS-CoV-2 Main Protease Inhibitors: From Nirmatrelvir to Future Perspectives, Biomolecules, doi:10.3390/biom13091339
Cooper, Zhang, Ibrahim, Zhang, Sun et al., Diastereomeric Resolution Yields Highly Potent Inhibitor of SARS-CoV-2 Main Protease, J. Med. Chem, doi:10.1021/acs.jmedchem.2c01131
Dai, Jochmans, Xie, Yang, Li et al., Synthesis, and Biological Evaluation of Peptidomimetic Aldehydes as Broad-Spectrum Inhibitors against Enterovirus and SARS-CoV-2, J. Med. Chem, doi:10.1021/acs.jmedchem.0c02258
Dai, Zhang, Jiang, Su, Li et al., Structure-Based Design of Antiviral Drug Candidates Targeting the SARS-CoV-2 Main Protease, Science, doi:10.1126/science.abb4489
De Oliveira, Da Rocha, Magalhães, Da Silva Mendes, Marinho et al., Computational Approach towards the Design of Artemisinin-Thymoquinone Hybrids against Main Protease of SARS-COV-2, Future J. Pharm. Sci, doi:10.1186/s43094-021-00334-z
Di Ciaula, Wang, Molina-Molina, Lunardi Baccetto, Calamita et al., Bile Acids and Cancer: Direct and Environmental-Dependent Effects, Ann. Hepatol, doi:10.5604/01.3001.0010.5501
Di Petrillo, Orrù, Fais, Fantini, Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review, Phytother. Res, doi:10.1002/ptr.7309
Dong, Fu, Yin, Cao, Li et al., A Review of Its Pharmacology, Toxicity and Pharmacokinetics, Phytother. Res, doi:10.1002/ptr.5631
El-Kalyoubi, Ragab, Abu Ali, Ammar, Seadawy et al., One-Pot Synthesis and Molecular Modeling Studies of New Bioactive Spiro-Oxindoles Based on Uracil Derivatives as SARS-CoV-2 Inhibitors Targeting RNA Polymerase and Spike Glycoprotein, Pharmaceuticals, doi:10.3390/ph15030376
Fröhlich, Ndreshkjana, Muenzner, Reiter, Hofmeister et al., Synthesis of Novel Hybrids of Thymoquinone and Artemisinin with High Activity and Selectivity Against Colon Cancer, ChemMedChem, doi:10.1002/cmdc.201600594
Fröhlich, Reiter, Saeed, Hutterer, Hahn et al., Synthesis of Thymoquinone-Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents, ACS Med. Chem. Lett, doi:10.1021/acsmedchemlett.7b00412
Gambino, Editorial: Development/Repurposing of Drugs to Tackle the Multiple Variants of SARS-CoV-2, Front. Drug Discov, doi:10.3389/fddsv.2023.1157688
George, Wells, Jenkins, Pulmonary Fibrosis and COVID-19: The Potential Role for Antifibrotic Therapy, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30225-3
Halford, The Path to Paxlovid, ACS Cent. Sci, doi:10.1021/acscentsci.2c00369
Hamming, Timens, Bulthuis, Lely, Navis et al., Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis, J. Pathol, doi:10.1002/path.1570
Hansa, Khan, Frangie, Gilmore, Shelton et al., A. 4-4-(Anilinomethyl)-3-[4-(Trifluoromethyl)Phenyl]-1H-Pyrazol-1-Ylbenzoic Acid Derivatives as Potent Anti-Gram-Positive Bacterial Agents, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2021.113402
Harmer, Gilbert, Borman, Clark, Quantitative mRNA Expression Profiling of ACE 2, a Novel Homologue of Angiotensin Converting Enzyme, FEBS Lett, doi:10.1016/S0014-5793(02)03640-2
Heravi, Zadsirjan, Prescribed Drugs Containing Nitrogen Heterocycles: An Overview, RSC Adv, doi:10.1039/D0RA09198G
Herrmann, Hahn, Wangen, Marschall, Tsogoeva, Anti-SARS-CoV-2 Inhibitory Profile of New Quinoline Compounds in Cell Culture-Based Infection Models, Chem. Eur. J, doi:10.1002/chem.202103861
Herrmann, Yaremenko, Çapcı, Struwe, Tailor et al., Synthesis and in Vitro Study of Artemisinin/Synthetic Peroxide-Based Hybrid Compounds against SARS-CoV-2 and Cancer, ChemMedChem, doi:10.1002/cmdc.202200005
Hoffman, Kania, Brothers, Davies, Ferre et al., Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19, J. Med. Chem, doi:10.1021/acs.jmedchem.0c01063
Hosseini Nasab, Azimian, Shim, Eom, Shah et al., Anticancer Evaluation, and Molecular Docking Studies of Thiazolyl-Pyrazoline Derivatives, Bioorg. Med. Chem. Lett, doi:10.1016/j.bmcl.2022.129105
Hurmach, Platonov, Prylutska, Scharff, Prylutskyy et al., C60 Fullerene against SARS-CoV-2 Coronavirus: An In Silico Insight, Sci. Rep, doi:10.1038/s41598-021-97268-6
Isakov, Koloda, Tikhonova, Kikalishvili, Krasavina et al., Pharmacokinetics of the New Hepatitis C Virus NS3 Protease Inhibitor Narlaprevir Following Single-Dose Use with or without Ritonavir in Patients with Liver Cirrhosis, Antimicrob. Agents Chemother, doi:10.1128/AAC.01044-16
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 Entry into Cells, Nat. Rev. Mol. Cell Biol, doi:10.1038/s41580-021-00418-x
Jiang, Feng, Chen, Nie, Chen et al., Discovery of Novel Nonpeptidic and Noncovalent Small Molecule 3CL pro Inhibitors as Anti-SARS-CoV-2 Drug Candidate, J. Med. Chem, doi:10.1021/acs.jmedchem.4c00739
Jiang, Feng, Zhang, Nie, Liu et al., Discovery of Novel Non-Peptidic and Non-Covalent Small-Molecule 3CLpro Inhibitors as Potential Candidate for COVID-19 Treatment, Signal Transduct. Target. Ther, doi:10.1038/s41392-023-01482-9
Jin, Du, Xu, Deng, Liu et al., Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Johnson, Strizki, Brown, Wan, Shamsuddin et al., Molnupiravir for the Treatment of COVID-19 in Immunocompromised Participants: Efficacy, Safety, and Virology Results from the Phase 3 Randomized, Placebo-Controlled MOVe-OUT Trial, Infection, doi:10.1007/s15010-022-01959-9
Jordheim, Durantel, Zoulim, Dumontet, Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases, Nat. Rev. Drug Discov, doi:10.1038/nrd4010
Kadil, Mouhcine, Filali, In Silico Investigation of the SARS CoV2 Protease with Thymoquinone, the Major Constituent of Nigella Sativa, Curr. Drug Discov. Technol, doi:10.2174/1570163817666200712164406
Kamal, Ramadan, Farraj, Bahig, Ezzat, The Pill of Recovery; Molnupiravir for Treatment of COVID-19 Patients; A Systematic Review, Saudi Pharm. J, doi:10.1016/j.jsps.2022.03.002
Kamath, Sunil, Ajees, Pai, Das, Some New Indole-Coumarin Hybrids; Synthesis, Anticancer and Bcl-2 Docking Studies, Bioorg. Chem, doi:10.1016/j.bioorg.2015.10.001
Kneller, Li, Phillips, Weiss, Zhang et al., Covalent Narlaprevir-and Boceprevir-Derived Hybrid Inhibitors of SARS-CoV-2 Main Protease, Nat. Commun, doi:10.1038/s41467-022-29915-z
Konno, Kobayashi, Senda, Funai, Seki et al., 3CL Protease Inhibitors with an Electrophilic Arylketone Moiety as Anti-SARS-CoV-2 Agents, J. Med. Chem, doi:10.1021/acs.jmedchem.1c00665
Kronenberger, Laufer, Pillaiyar, COVID-19 Therapeutics: Small-Molecule Drug Development Targeting SARS-CoV-2 Main Protease, Drug Discov. Today, doi:10.1016/j.drudis.2023.103579
Kumar, Tan, Wang, Lin, Liang, Synthesis and Evaluation of SARS-CoV and MERS-CoV 3C-like Protease Inhibitors, Bioorg. Med. Chem, doi:10.1016/j.bmc.2016.05.013
Kumari, Dhillon, Rani, Chahal, Aneja et al., Development in the Synthesis of Bioactive Thiazole-Based Heterocyclic Hybrids Utilizing Phenacyl Bromide, ACS Omega, doi:10.1021/acsomega.3c10299
Lee, Hur, Sung, The Effect of Artemisinin on Inflammation-Associated Lymphangiogenesis in Experimental Acute Colitis, Int. J. Mol. Sci, doi:10.3390/ijms21218068
Lemos, Makowski, Almagro, Tolón, Rodríguez et al., Synthesis of [60]Fullerene Hybrids Endowed with Steroids and Monosaccharides: Theoretical Underpinning as Promising anti-SARS-CoV-2 Agents, Eur. J. Org. Chem, doi:10.1002/ejoc.202201301
Lengerli, Ibis, Nural, Banoglu, The 1,2,3-Triazole 'All-in-One' Ring System in Drug Discovery: A Good Bioisostere, a Good Pharmacophore, a Good Linker, and a Versatile Synthetic Tool, Expert Opin. Drug Discov, doi:10.1080/17460441.2022.2129613
Li, Touret, De Lamballerie, Nguyen, Laurent et al., Hybrid Molecules Based on an Emodin Scaffold. Synthesis and Activity against SARS-CoV-2 and Plasmodium, Org. Biomol. Chem, doi:10.1039/D3OB01122D
Liang, Tian, Liu, Hui, Qiao et al., A Promising Antiviral Candidate Drug for the COVID-19 Pandemic: A Mini-Review of Remdesivir, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2020.112527
Lin, Li, Zhang, Liu, Mu et al., Ceftazidime Is a Potential Drug to Inhibit SARS-CoV-2 Infection in Vitro by Blocking Spike Protein-ACE2 Interaction, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00619-y
Lu, Zhu, Fox, Gao, Stanley et al., SARS-CoV-2 down-Regulates ACE2 through Lysosomal Degradation, Mol. Biol. Cell, doi:10.1091/mbc.E22-02-0045
Lv, Cano, Jia, Drag, Huang et al., Targeting SARS-CoV-2 Proteases for COVID-19 Antiviral Development, Front. Chem, doi:10.3389/fchem.2021.819165
Malebari, Ahmed, Ihmaid, Omar, Muhammad et al., Exploring the Dual Effect of Novel 1,4-Diarylpyranopyrazoles as Antiviral and Anti-Inflammatory for the Management of SARS-CoV-2 and Associated Inflammatory Symptoms, Bioorg. Chem, doi:10.1016/j.bioorg.2022.106255
Malin, Weibel, Gruell, Kreuzberger, Stegemann et al., Efficacy and Safety of Molnupiravir for the Treatment of SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis, J. Antimicrob. Chemother, doi:10.1093/jac/dkad132
Mangalmurti, Hunter, Cytokine Storms: Understanding COVID-19, Immunity, doi:10.1016/j.immuni.2020.06.017
Mansour, Aboulmagd, Abdel-Rahman, Quinazoline-Schiff Base Conjugates: In Silico Study and ADMET Predictions as Multi-Target Inhibitors of Coronavirus (SARS-CoV-2) Proteins, RSC Adv, doi:10.1039/D0RA06424F
Marchesi, Chinaglia, Capobianco, Marchetti, Huang et al., Dihydroartemisinin-Bile Acid Hybridization as an Effective Approach to Enhance Dihydroartemisinin Anticancer Activity, ChemMedChem, doi:10.1002/cmdc.201800756
Marchesi, Gentili, Bortolotti, Preti, Marchetti et al., Dihydroartemisinin-Ursodeoxycholic Bile Acid Hybrids in the Fight against SARS-CoV-2, ACS Omega, doi:10.1021/acsomega.3c07034
Marchesi, Perrone, Navacchia, Molecular Hybridization as a Strategy for Developing Artemisinin-Derived Anticancer Candidates, Pharmaceutics, doi:10.3390/pharmaceutics15092185
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications, Clin. Pharmacol. Ther, doi:10.1002/cpt.2646
Mehrabadi, Hemmati, Tashakor, Homaei, Yousefzadeh et al., Induced Dysregulation of ACE2 by SARS-CoV-2 Plays a Key Role in COVID-19 Severity, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111363
Meng, Han, Zhang, Guo, Cui et al., Synthesis and Anti-Inflammatory Activity of N-Phthalimidomethyl 2,3-Dideoxy-and 2,3-Unsaturated Glycosides, Carbohydr. Res, doi:10.1016/j.carres.2007.03.009
Metwally, Elgemeie, Fahmy, Synthesis and Biological Evaluation of Benzothiazolyl-Pyridine Hybrids as New Antiviral Agents against H5N1 Bird Flu and SARS-COV-2 Viruses, ACS Omega, doi:10.1021/acsomega.3c01987
Meunier, Hybrid Molecules with a Dual Mode of Action: Dream or Reality?, Acc. Chem. Res, doi:10.1021/ar7000843
Monica, Bono, Lauria, Martorana, Targeting SARS-CoV-2 Main Protease for Treatment of COVID-19: Covalent Inhibitors Structure-Activity Relationship Insights and Evolution Perspectives, J. Med. Chem, doi:10.1021/acs.jmedchem.2c01005
Musa, Abulkhair, Aljuhani, Rezki, Abdelgawad et al., Phenylpyrazolone-1,2,3-Triazole Hybrids as Potent Antiviral Agents with Promising SARS-CoV-2 Main Protease Inhibition Potential, Pharmaceuticals, doi:10.3390/ph16030463
Navacchia, Marchesi, Mari, Chinaglia, Gallerani et al., Rational Design of Nucleoside-Bile Acid Conjugates Incorporating a Triazole Moiety for Anticancer Evaluation and SAR Exploration, Molecules, doi:10.3390/molecules22101710
Navacchia, Marchesi, Perrone, Bile Acid Conjugates with Anticancer Activity: Most Recent Research, Molecules, doi:10.3390/molecules26010025
Nocentini, Capasso, Supuran, Perspectives on the Design and Discovery of α-Ketoamide Inhibitors for the Treatment of Novel Coronavirus: Where Do We Stand and Where Do We Go?, Expert Opin. Drug Discov, doi:10.1080/17460441.2022.2052847
Othman, Gad-Elkareem, El-Naggar, Nossier, Amr, Novel Phthalimide Based Analogues: Design, Synthesis, Biological Evaluation, and Molecular Docking Studies, J. Enzyme Inhib. Med. Chem, doi:10.1080/14756366.2019.1637861
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19, Science
Perrone, Marchesi, Preti, Navacchia, Modified Nucleosides, Nucleotides and Nucleic Acids via Click Azide-Alkyne Cycloaddition for Pharmacological Applications, Molecules, doi:10.3390/molecules26113100
Ramajayam, Tan, Liu, Liang, Synthesis and Evaluation of Pyrazolone Compounds as SARS-Coronavirus 3C-like Protease Inhibitors, Bioorg. Med. Chem, doi:10.1016/j.bmc.2010.09.050
Romano, Ruggiero, Squeglia, Maga, Berisio, A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping, Cells, doi:10.3390/cells9051267
Saha, Bonnier, Chong, Antimalarials as Antivirals for COVID-19: Believe It or Not!, Am. J. Med. Sci, doi:10.1016/j.amjms.2020.08.019
Seliem, Synthesis and Virtual Screening of Pyrazolothiazole Conjugates as Promising SARS-CoV-2 Inhibitors, ChemistrySelect, doi:10.1002/slct.202300605
Seltzer, Linking ACE2 and Angiotensin II to Pulmonary Immunovascular Dysregulation in SARS-CoV-2 Infection, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.09.041
Shang, Wan, Luo, Ye, Geng et al., Cell Entry Mechanisms of SARS-CoV-2, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2003138117
Sharma, Das, Kumar Mehta, Gupta, Venugopala et al., Recent Insight into the Biological Activities and SAR of Quinolone Derivatives as Multifunctional Scaffold, Bioorg. Med. Chem
Soliman, Hamoda, Nayak, Mostafa, Hamdy, Novel Compounds with Dual Inhibition Activity against SARS-CoV-2 Critical Enzymes RdRp and Human TMPRSS2, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2024.116671
Stefanelli, Corona, Cerchia, Cassese, Improta et al., Broad-Spectrum Coronavirus 3C-like Protease Peptidomimetic Inhibitors Effectively Block SARS-CoV-2 Replication in Cells: Design, Synthesis, Biological Evaluation, and X-Ray Structure Determination, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115311
Tang, Li, Yuan, Zhang, Zou et al., Network Pharmacology-Based Predictions of Active Components and Pharmacological Mechanisms of Artemisia annua L. for the Treatment of the Novel Corona Virus Disease 2019 (COVID-19), BMC Complement. Med. Ther, doi:10.1186/s12906-022-03523-2
Thuy, Bao, Moon, Ursodeoxycholic Acid Ameliorates Cell Migration Retarded by the SARS-CoV-2 Spike Protein in BEAS-2B Human Bronchial Epithelial Cells, Biomed. Pharmacother, doi:10.1016/j.biopha.2022.113021
Tian, Liu, Liang, Xin, Xie et al., An Update Review of Emerging Small-Molecule Therapeutic Options for COVID-19, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111313
Tu, The Discovery of Artemisinin (Qinghaosu) and Gifts from Chinese Medicine, Nat. Med, doi:10.1038/nm.2471
Ullrich, Nitsche, The SARS-CoV-2 Main Protease as Drug Target, Bioorg. Med. Chem. Lett, doi:10.1016/j.bmcl.2020.127377
Vishwanath, Shete-Aich, Honnegowda, Anand, Chidambaram et al., Discovery of Hybrid Thiouracil-Coumarin Conjugates as Potential Novel Anti-SARS-CoV-2 Agents Targeting the Virus's Polymerase "RdRp" as a Confirmed Interacting Biomolecule, ACS Omega, doi:10.1021/acsomega.3c02079
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein, Cell, doi:10.1016/j.cell.2020.02.058
Wang, Yang, Shan, Zhao, Bai et al., Paxlovid for the Treatment of COVID-19: A Systematic Review and Meta-Analysis, J. Infect. Dev. Ctries, doi:10.3855/jidc.19202
Wu, Peng, Huang, Ding, Wang et al., Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China, Cell Host Microbe, doi:10.1016/j.chom.2020.02.001
Wyman, Girgis, Surapaneni, Moore, Abo Shama et al., Synthesis of Potential Antiviral Agents for SARS-CoV-2 Using Molecular Hybridization Approach, Molecules, doi:10.3390/molecules27185923
Xu, Zhao, Liu, 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2019.111700
Yadav, Kaushik, Kumar, Kumar, Phthalimide/Naphthalimide Containing 1,2,3-Triazole Hybrids: Synthesis and Antimicrobial Evaluation, J. Mol. Struct, doi:10.1016/j.molstruc.2022.134688
Yang, Li, Jin, Song, Liu et al., Synthesis and Bioactivity of 4-Alkyl (Aryl)Thioquinazoline Derivatives, Bioorg. Med. Chem. Lett, doi:10.1016/j.bmcl.2007.01.101
Yuan, Ma, Xie, Li, Su et al., The Role of Cell Death in SARS-CoV-2 Infection, Signal Transduct. Target. Ther, doi:10.1038/s41392-023-01580-8
Zhan, Ta, Tang, Hua, Wang et al., Potential Antiviral Activity of Isorhamnetin against SARS-CoV-2 Spike Pseudotyped Virus in Vitro, Drug Dev. Res, doi:10.1002/ddr.21815
Zhang, Chen, Yang, A Review on Recent Developments of Indole-Containing Antiviral Agents, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2014.10.065
Zhang, Lin, Sun, Curth, Drosten et al., Crystal Structure of SARS-CoV-2 Main Protease Provides a Basis for Design of Improved α-Ketoamide Inhibitors, Science, doi:10.1126/science.abb3405
Zhang, Wang, Zhao, Wang, Zhu et al., Synthesis and in Vitro Anti-Influenza A Virus Evaluation of Novel Quinazoline Derivatives Containing S-Acetamide and NH-Acetamide Moieties at C-4, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2020.112706
Zhao, Dai, Li, Wang, Tian et al., Pyrazolone Structural Motif in Medicinal Chemistry: Retrospect and Prospect, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2019.111893
Zhao, Zhang, Wang, Liu, Lei et al., Quinoline and Quinazoline Derivatives Inhibit Viral RNA Synthesis by SARS-CoV-2 RdRp, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00083
Çapcı Karagöz, Reiter, Seo, Gruber, Hahn et al., Access to New Highly Potent Antileukemia, Antiviral and Antimalarial Agents via Hybridization of Natural Products (Homo)Egonol, Thymoquinone and Artemisinin, Bioorg. Med. Chem, doi:10.1016/j.bmc.2018.05.041
DOI record:
{
"DOI": "10.3390/molecules29225403",
"ISSN": [
"1420-3049"
],
"URL": "http://dx.doi.org/10.3390/molecules29225403",
"abstract": "<jats:p>The advantages of a treatment modality that combines two or more therapeutic agents with different mechanisms of action encourage the study of hybrid functional compounds for pharmacological applications. Molecular hybridization, resulting from a covalent combination of two or more pharmacophore units, has emerged as a promising approach to overcome several issues and has also been explored for the design of new drugs for COVID-19 treatment. In this review, we presented an overview of small-molecule hybrids from both natural products and synthetic sources reported in the literature to date with potential antiviral anti-SARS-CoV-2 activity.</jats:p>",
"alternative-id": [
"molecules29225403"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7175-1504",
"affiliation": [
{
"name": "Institute for Organic Synthesis and Photoreactivity (ISOF), National Research Council of Italy (CNR), 40129 Bologna, Italy"
}
],
"authenticated-orcid": false,
"family": "Navacchia",
"given": "Maria Luisa",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-8049-0369",
"affiliation": [
{
"name": "Institute for Organic Synthesis and Photoreactivity (ISOF), National Research Council of Italy (CNR), 40129 Bologna, Italy"
}
],
"authenticated-orcid": false,
"family": "Cinti",
"given": "Caterina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5402-4732",
"affiliation": [
{
"name": "Department of Chemical, Pharmaceutical and Agricultural Sciences, University of Ferrara, 44121 Ferrara, Italy"
}
],
"authenticated-orcid": false,
"family": "Marchesi",
"given": "Elena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5189-011X",
"affiliation": [
{
"name": "Department of Environmental and Prevention Sciences, University of Ferrara, 44121 Ferrara, Italy"
}
],
"authenticated-orcid": false,
"family": "Perrone",
"given": "Daniela",
"sequence": "additional"
}
],
"container-title": "Molecules",
"container-title-short": "Molecules",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T12:51:15Z",
"timestamp": 1732020675000
},
"deposited": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T12:54:11Z",
"timestamp": 1732020851000
},
"indexed": {
"date-parts": [
[
2024,
11,
20
]
],
"date-time": "2024-11-20T05:30:38Z",
"timestamp": 1732080638210,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issue": "22",
"issued": {
"date-parts": [
[
2024,
11,
15
]
]
},
"journal-issue": {
"issue": "22",
"published-online": {
"date-parts": [
[
2024,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
15
]
],
"date-time": "2024-11-15T00:00:00Z",
"timestamp": 1731628800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1420-3049/29/22/5403/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "5403",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
11,
15
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
15
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "World Health Organization (2024). COVID-19 Epidemiological Update—17 September 2024, World Health Organization."
},
{
"DOI": "10.1080/14760584.2021.1903879",
"article-title": "Effects of SARS-CoV-2 Variants on Vaccine Efficacy and Response Strategies",
"author": "Bian",
"doi-asserted-by": "crossref",
"first-page": "365",
"journal-title": "Expert Rev. Vaccines",
"key": "ref_2",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.111313",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Tian, D., Liu, Y., Liang, C., Xin, L., Xie, X., Zhang, D., Wan, M., Li, H., Fu, X., and Liu, H. (2021). An Update Review of Emerging Small-Molecule Therapeutic Options for COVID-19. Biomed. Pharmacother., 137."
},
{
"DOI": "10.1016/j.ejmech.2020.112527",
"article-title": "A Promising Antiviral Candidate Drug for the COVID-19 Pandemic: A Mini-Review of Remdesivir",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "112527",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_4",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.1016/j.jsps.2022.03.002",
"article-title": "The Pill of Recovery; Molnupiravir for Treatment of COVID-19 Patients; A Systematic Review",
"author": "Kamal",
"doi-asserted-by": "crossref",
"first-page": "508",
"journal-title": "Saudi Pharm. J.",
"key": "ref_5",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19",
"author": "Owen",
"doi-asserted-by": "crossref",
"first-page": "1586",
"journal-title": "Science",
"key": "ref_6",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1021/acscentsci.2c00369",
"article-title": "The Path to Paxlovid",
"author": "Halford",
"doi-asserted-by": "crossref",
"first-page": "405",
"journal-title": "ACS Cent. Sci.",
"key": "ref_7",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.3855/jidc.19202",
"article-title": "Paxlovid for the Treatment of COVID-19: A Systematic Review and Meta-Analysis",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1169",
"journal-title": "J. Infect. Dev. Ctries.",
"key": "ref_8",
"volume": "18",
"year": "2024"
},
{
"DOI": "10.1093/jac/dkad132",
"article-title": "Efficacy and Safety of Molnupiravir for the Treatment of SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis",
"author": "Malin",
"doi-asserted-by": "crossref",
"first-page": "1586",
"journal-title": "J. Antimicrob. Chemother.",
"key": "ref_9",
"volume": "78",
"year": "2023"
},
{
"DOI": "10.1007/s15010-022-01959-9",
"article-title": "Molnupiravir for the Treatment of COVID-19 in Immunocompromised Participants: Efficacy, Safety, and Virology Results from the Phase 3 Randomized, Placebo-Controlled MOVe-OUT Trial",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "1273",
"journal-title": "Infection",
"key": "ref_10",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the Management of Drug–Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "ref_11",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.3389/fddsv.2023.1157688",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Gambino, D. (2023). Editorial: Development/Repurposing of Drugs to Tackle the Multiple Variants of SARS-CoV-2. Front. Drug Discov., 3."
},
{
"DOI": "10.1021/ar7000843",
"article-title": "Hybrid Molecules with a Dual Mode of Action: Dream or Reality?",
"author": "Meunier",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Acc. Chem. Res.",
"key": "ref_13",
"volume": "41",
"year": "2008"
},
{
"DOI": "10.3390/molecules26113100",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Perrone, D., Marchesi, E., Preti, L., and Navacchia, M.L. (2021). Modified Nucleosides, Nucleotides and Nucleic Acids via Click Azide-Alkyne Cycloaddition for Pharmacological Applications. Molecules, 26."
},
{
"DOI": "10.1016/j.ejmech.2019.111700",
"article-title": "1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "111700",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_15",
"volume": "183",
"year": "2019"
},
{
"DOI": "10.3390/molecules22101710",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Navacchia, M., Marchesi, E., Mari, L., Chinaglia, N., Gallerani, E., Gavioli, R., Capobianco, M., and Perrone, D. (2017). Rational Design of Nucleoside–Bile Acid Conjugates Incorporating a Triazole Moiety for Anticancer Evaluation and SAR Exploration. Molecules, 22."
},
{
"DOI": "10.3390/pharmaceutics15092185",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Marchesi, E., Perrone, D., and Navacchia, M.L. (2023). Molecular Hybridization as a Strategy for Developing Artemisinin-Derived Anticancer Candidates. Pharmaceutics, 15."
},
{
"DOI": "10.3390/molecules26010025",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Navacchia, M.L., Marchesi, E., and Perrone, D. (2020). Bile Acid Conjugates with Anticancer Activity: Most Recent Research. Molecules, 26."
},
{
"DOI": "10.1016/j.chom.2020.02.001",
"article-title": "Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "325",
"journal-title": "Cell Host Microbe",
"key": "ref_19",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.20944/preprints202004.0510.v1",
"doi-asserted-by": "crossref",
"key": "ref_20",
"unstructured": "Romano, M., Ruggiero, A., Squeglia, F., Maga, G., and Berisio, R. (2020). A Structural View of SARS-CoV-2 RNA Replication Machinery: RNA Synthesis, Proofreading and Final Capping. Cells, 9."
},
{
"DOI": "10.3389/fchem.2021.819165",
"doi-asserted-by": "crossref",
"key": "ref_21",
"unstructured": "Lv, Z., Cano, K.E., Jia, L., Drag, M., Huang, T.T., and Olsen, S.K. (2022). Targeting SARS-CoV-2 Proteases for COVID-19 Antiviral Development. Front. Chem., 9."
},
{
"DOI": "10.1016/j.bmcl.2020.127377",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Ullrich, S., and Nitsche, C. (2020). The SARS-CoV-2 Main Protease as Drug Target. Bioorg. Med. Chem. Lett., 30."
},
{
"DOI": "10.1021/acs.jmedchem.2c01005",
"article-title": "Targeting SARS-CoV-2 Main Protease for Treatment of COVID-19: Covalent Inhibitors Structure–Activity Relationship Insights and Evolution Perspectives",
"author": "Bono",
"doi-asserted-by": "crossref",
"first-page": "12500",
"journal-title": "J. Med. Chem.",
"key": "ref_23",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1016/j.coviro.2024.101411",
"article-title": "Early Signaling Pathways in Virus-Infected Cells",
"author": "Bonhomme",
"doi-asserted-by": "crossref",
"first-page": "101411",
"journal-title": "Curr. Opin. Virol.",
"key": "ref_24",
"volume": "66",
"year": "2024"
},
{
"DOI": "10.1016/j.immuni.2020.06.017",
"article-title": "Cytokine Storms: Understanding COVID-19",
"author": "Mangalmurti",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "Immunity",
"key": "ref_25",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2021.111363",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Mehrabadi, M.E., Hemmati, R., Tashakor, A., Homaei, A., Yousefzadeh, M., Hemati, K., and Hosseinkhani, S. (2021). Induced Dysregulation of ACE2 by SARS-CoV-2 Plays a Key Role in COVID-19 Severity. Biomed. Pharmacother., 137."
},
{
"DOI": "10.1038/s41392-023-01580-8",
"article-title": "The Role of Cell Death in SARS-CoV-2 Infection",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "357",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_27",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2021.728896",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Barhoumi, T., Alghanem, B., Shaibah, H., Mansour, F.A., Alamri, H.S., Akiel, M.A., Alroqi, F., and Boudjelal, M. (2021). SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). Front. Immunol., 12."
},
{
"DOI": "10.1002/path.1570",
"article-title": "Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis",
"author": "Hamming",
"doi-asserted-by": "crossref",
"first-page": "631",
"journal-title": "J. Pathol.",
"key": "ref_29",
"volume": "203",
"year": "2004"
},
{
"DOI": "10.1016/S0014-5793(02)03640-2",
"article-title": "Quantitative mRNA Expression Profiling of ACE 2, a Novel Homologue of Angiotensin Converting Enzyme",
"author": "Harmer",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "FEBS Lett.",
"key": "ref_30",
"volume": "532",
"year": "2002"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"article-title": "Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein",
"author": "Walls",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Cell",
"key": "ref_31",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2003138117",
"article-title": "Cell Entry Mechanisms of SARS-CoV-2",
"author": "Shang",
"doi-asserted-by": "crossref",
"first-page": "11727",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_32",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1038/s41580-021-00418-x",
"article-title": "Mechanisms of SARS-CoV-2 Entry into Cells",
"author": "Jackson",
"doi-asserted-by": "crossref",
"first-page": "3",
"journal-title": "Nat. Rev. Mol. Cell Biol.",
"key": "ref_33",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.09.041",
"article-title": "Linking ACE2 and Angiotensin II to Pulmonary Immunovascular Dysregulation in SARS-CoV-2 Infection",
"author": "Seltzer",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_34",
"volume": "101",
"year": "2020"
},
{
"DOI": "10.1091/mbc.E22-02-0045",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Lu, Y., Zhu, Q., Fox, D.M., Gao, C., Stanley, S.A., and Luo, K. (2022). SARS-CoV-2 down-Regulates ACE2 through Lysosomal Degradation. Mol. Biol. Cell, 33."
},
{
"DOI": "10.4196/kjpp.2018.22.4.447",
"article-title": "Angiotensin-(1-9) Ameliorates Pulmonary Arterial Hypertension via Angiotensin Type II Receptor",
"author": "Cha",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Korean J. Physiol. Pharmacol.",
"key": "ref_36",
"volume": "22",
"year": "2018"
},
{
"DOI": "10.1016/S2213-2600(20)30225-3",
"article-title": "Pulmonary Fibrosis and COVID-19: The Potential Role for Antifibrotic Therapy",
"author": "George",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Lancet Respir. Med.",
"key": "ref_37",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.amjms.2020.08.019",
"article-title": "Antimalarials as Antivirals for COVID-19: Believe It or Not!",
"author": "Saha",
"doi-asserted-by": "crossref",
"first-page": "618",
"journal-title": "Am. J. Med. Sci.",
"key": "ref_38",
"volume": "360",
"year": "2020"
},
{
"DOI": "10.1038/nm.2471",
"article-title": "The Discovery of Artemisinin (Qinghaosu) and Gifts from Chinese Medicine",
"author": "Tu",
"doi-asserted-by": "crossref",
"first-page": "1217",
"journal-title": "Nat. Med.",
"key": "ref_39",
"volume": "17",
"year": "2011"
},
{
"DOI": "10.3390/ijms21218068",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Lee, A.S., Hur, H.J., and Sung, M.J. (2020). The Effect of Artemisinin on Inflammation-Associated Lymphangiogenesis in Experimental Acute Colitis. Int. J. Mol. Sci., 21."
},
{
"DOI": "10.1002/ddr.21815",
"article-title": "Potential Antiviral Activity of Isorhamnetin against SARS-CoV-2 Spike Pseudotyped Virus in Vitro",
"author": "Zhan",
"doi-asserted-by": "crossref",
"first-page": "1124",
"journal-title": "Drug Dev. Res.",
"key": "ref_41",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7309",
"article-title": "Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review",
"author": "Fais",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "Phytother. Res.",
"key": "ref_42",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1186/s12906-022-03523-2",
"doi-asserted-by": "crossref",
"key": "ref_43",
"unstructured": "Tang, Y., Li, X., Yuan, Y., Zhang, H., Zou, Y., Xu, Z., Xu, Q., Song, J., Deng, C., and Wang, Q. (2022). Network Pharmacology-Based Predictions of Active Components and Pharmacological Mechanisms of Artemisia annua L. for the Treatment of the Novel Corona Virus Disease 2019 (COVID-19). BMC Complement. Med. Ther., 22."
},
{
"DOI": "10.1021/acsinfecdis.0c00522",
"article-title": "Anti-SARS-CoV-2 Potential of Artemisinins In Vitro",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "2524",
"journal-title": "ACS Infect. Dis.",
"key": "ref_44",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.5604/01.3001.0010.5501",
"article-title": "Bile Acids and Cancer: Direct and Environmental-Dependent Effects",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "S87",
"journal-title": "Ann. Hepatol.",
"key": "ref_45",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1080/07391102.2020.1775129",
"article-title": "Molecular Docking, Simulation and MM-PBSA Studies of Nigella Sativa Compounds: A Computational Quest to Identify Potential Natural Antiviral for COVID-19 Treatment",
"author": "Ahmad",
"doi-asserted-by": "crossref",
"first-page": "4225",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "ref_46",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.2174/1570163817666200712164406",
"article-title": "In Silico Investigation of the SARS CoV2 Protease with Thymoquinone, the Major Constituent of Nigella Sativa",
"author": "Kadil",
"doi-asserted-by": "crossref",
"first-page": "570",
"journal-title": "Curr. Drug Discov. Technol.",
"key": "ref_47",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1021/acs.jmedchem.0c01063",
"article-title": "Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19",
"author": "Hoffman",
"doi-asserted-by": "crossref",
"first-page": "12725",
"journal-title": "J. Med. Chem.",
"key": "ref_48",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1002/cmdc.201600594",
"article-title": "Synthesis of Novel Hybrids of Thymoquinone and Artemisinin with High Activity and Selectivity Against Colon Cancer",
"author": "Ndreshkjana",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "ChemMedChem",
"key": "ref_49",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1021/acsmedchemlett.7b00412",
"article-title": "Synthesis of Thymoquinone–Artemisinin Hybrids: New Potent Antileukemia, Antiviral, and Antimalarial Agents",
"author": "Reiter",
"doi-asserted-by": "crossref",
"first-page": "534",
"journal-title": "ACS Med. Chem. Lett.",
"key": "ref_50",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.bmc.2018.05.041",
"article-title": "Access to New Highly Potent Antileukemia, Antiviral and Antimalarial Agents via Hybridization of Natural Products (Homo)Egonol, Thymoquinone and Artemisinin",
"author": "Reiter",
"doi-asserted-by": "crossref",
"first-page": "3610",
"journal-title": "Bioorg. Med. Chem.",
"key": "ref_51",
"volume": "26",
"year": "2018"
},
{
"DOI": "10.1186/s43094-021-00334-z",
"article-title": "Computational Approach towards the Design of Artemisinin–Thymoquinone Hybrids against Main Protease of SARS-COV-2",
"author": "Marinho",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Future J. Pharm. Sci.",
"key": "ref_52",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1101/2020.02.27.968008",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Bzówka, M., Mitusińska, K., Raczyńska, A., Samol, A., Tuszyński, J.A., and Góra, A. (2020). Structural and Evolutionary Analysis Indicate That the SARS-CoV-2 Mpro Is a Challenging Target for Small-Molecule Inhibitor Design. Int. J. Mol. Sci., 21."
},
{
"DOI": "10.1002/cmdc.202200005",
"article-title": "Synthesis and in Vitro Study of Artemisinin/Synthetic Peroxide-Based Hybrid Compounds against SARS-CoV-2 and Cancer",
"author": "Herrmann",
"doi-asserted-by": "crossref",
"first-page": "e202200005",
"journal-title": "ChemMedChem",
"key": "ref_54",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2014.07.044",
"article-title": "A Review on Anticancer Potential of Bioactive Heterocycle Quinoline",
"author": "Afzal",
"doi-asserted-by": "crossref",
"first-page": "871",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_55",
"volume": "97",
"year": "2015"
},
{
"DOI": "10.1021/acsinfecdis.1c00083",
"article-title": "Quinoline and Quinazoline Derivatives Inhibit Viral RNA Synthesis by SARS-CoV-2 RdRp",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "1535",
"journal-title": "ACS Infect. Dis.",
"key": "ref_56",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1002/cmdc.201800756",
"article-title": "Dihydroartemisinin–Bile Acid Hybridization as an Effective Approach to Enhance Dihydroartemisinin Anticancer Activity",
"author": "Marchesi",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "ChemMedChem",
"key": "ref_57",
"volume": "14",
"year": "2019"
},
{
"DOI": "10.1021/acsomega.3c07034",
"article-title": "Dihydroartemisinin-Ursodeoxycholic Bile Acid Hybrids in the Fight against SARS-CoV-2",
"author": "Marchesi",
"doi-asserted-by": "crossref",
"first-page": "45078",
"journal-title": "ACS Omega",
"key": "ref_58",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"article-title": "FXR Inhibition May Protect from SARS-CoV-2 Infection by Reducing ACE2",
"author": "Brevini",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "Nature",
"key": "ref_59",
"volume": "615",
"year": "2023"
},
{
"DOI": "10.1016/j.biopha.2022.113021",
"doi-asserted-by": "crossref",
"key": "ref_60",
"unstructured": "Thuy, P.X., Bao, T.D.D., and Moon, E.-Y. (2022). Ursodeoxycholic Acid Ameliorates Cell Migration Retarded by the SARS-CoV-2 Spike Protein in BEAS-2B Human Bronchial Epithelial Cells. Biomed. Pharmacother., 150."
},
{
"key": "ref_61",
"unstructured": "(2024, October 17). The Application of Ursodeoxycholic Acid for the Prevention of SARS-CoV-2 Infection (COVID-19); ClinicalTrials.Gov Identifier: NCT05659654, Available online: https://clinicaltrials.gov/study/NCT05659654."
},
{
"DOI": "10.1038/s41586-020-2223-y",
"article-title": "Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Nature",
"key": "ref_62",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.1c00665",
"article-title": "3CL Protease Inhibitors with an Electrophilic Arylketone Moiety as Anti-SARS-CoV-2 Agents",
"author": "Konno",
"doi-asserted-by": "crossref",
"first-page": "2926",
"journal-title": "J. Med. Chem.",
"key": "ref_63",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1016/j.drudis.2023.103579",
"article-title": "COVID-19 Therapeutics: Small-Molecule Drug Development Targeting SARS-CoV-2 Main Protease",
"author": "Kronenberger",
"doi-asserted-by": "crossref",
"first-page": "103579",
"journal-title": "Drug Discov. Today",
"key": "ref_64",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.20944/preprints202308.0055.v1",
"doi-asserted-by": "crossref",
"key": "ref_65",
"unstructured": "Citarella, A., Dimasi, A., Moi, D., Passarella, D., Scala, A., Piperno, A., and Micale, N. (2023). Recent Advances in SARS-CoV-2 Main Protease Inhibitors: From Nirmatrelvir to Future Perspectives. Biomolecules, 13."
},
{
"DOI": "10.1080/17460441.2022.2052847",
"article-title": "Perspectives on the Design and Discovery of α-Ketoamide Inhibitors for the Treatment of Novel Coronavirus: Where Do We Stand and Where Do We Go?",
"author": "Nocentini",
"doi-asserted-by": "crossref",
"first-page": "547",
"journal-title": "Expert Opin. Drug Discov.",
"key": "ref_66",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-29915-z",
"article-title": "Covalent Narlaprevir- and Boceprevir-Derived Hybrid Inhibitors of SARS-CoV-2 Main Protease",
"author": "Kneller",
"doi-asserted-by": "crossref",
"first-page": "2268",
"journal-title": "Nat. Commun.",
"key": "ref_67",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1021/ml9000276",
"article-title": "Discovery of Narlaprevir (SCH 900518): A Potent, Second Generation HCV NS3 Serine Protease Inhibitor",
"author": "Arasappan",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "ACS Med. Chem. Lett.",
"key": "ref_68",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.1128/AAC.01044-16",
"article-title": "Pharmacokinetics of the New Hepatitis C Virus NS3 Protease Inhibitor Narlaprevir Following Single-Dose Use with or without Ritonavir in Patients with Liver Cirrhosis",
"author": "Isakov",
"doi-asserted-by": "crossref",
"first-page": "7098",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_69",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.1126/science.abb4489",
"article-title": "Structure-Based Design of Antiviral Drug Candidates Targeting the SARS-CoV-2 Main Protease",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "1331",
"journal-title": "Science",
"key": "ref_70",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.0c02258",
"article-title": "Design, Synthesis, and Biological Evaluation of Peptidomimetic Aldehydes as Broad-Spectrum Inhibitors against Enterovirus and SARS-CoV-2",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "2794",
"journal-title": "J. Med. Chem.",
"key": "ref_71",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2023.115311",
"article-title": "Broad-Spectrum Coronavirus 3C-like Protease Peptidomimetic Inhibitors Effectively Block SARS-CoV-2 Replication in Cells: Design, Synthesis, Biological Evaluation, and X-Ray Structure Determination",
"author": "Stefanelli",
"doi-asserted-by": "crossref",
"first-page": "115311",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_72",
"volume": "253",
"year": "2023"
},
{
"DOI": "10.1126/science.abb3405",
"article-title": "Crystal Structure of SARS-CoV-2 Main Protease Provides a Basis for Design of Improved α-Ketoamide Inhibitors",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Science",
"key": "ref_73",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.2c01131",
"article-title": "Diastereomeric Resolution Yields Highly Potent Inhibitor of SARS-CoV-2 Main Protease",
"author": "Cooper",
"doi-asserted-by": "crossref",
"first-page": "13328",
"journal-title": "J. Med. Chem.",
"key": "ref_74",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1039/D0RA09198G",
"article-title": "Prescribed Drugs Containing Nitrogen Heterocycles: An Overview",
"author": "Heravi",
"doi-asserted-by": "crossref",
"first-page": "44247",
"journal-title": "RSC Adv.",
"key": "ref_75",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.ejmech.2020.112652",
"article-title": "An Insight on Medicinal Attributes of 1,2,4-Triazoles",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "112652",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_76",
"volume": "205",
"year": "2020"
},
{
"DOI": "10.1080/17460441.2022.2129613",
"article-title": "The 1,2,3-Triazole ‘All-in-One’ Ring System in Drug Discovery: A Good Bioisostere, a Good Pharmacophore, a Good Linker, and a Versatile Synthetic Tool",
"author": "Lengerli",
"doi-asserted-by": "crossref",
"first-page": "1209",
"journal-title": "Expert Opin. Drug Discov.",
"key": "ref_77",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2019.111893",
"article-title": "Pyrazolone Structural Motif in Medicinal Chemistry: Retrospect and Prospect",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "111893",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_78",
"volume": "186",
"year": "2020"
},
{
"DOI": "10.1016/j.bmc.2010.09.050",
"article-title": "Synthesis and Evaluation of Pyrazolone Compounds as SARS-Coronavirus 3C-like Protease Inhibitors",
"author": "Ramajayam",
"doi-asserted-by": "crossref",
"first-page": "7849",
"journal-title": "Bioorg. Med. Chem.",
"key": "ref_79",
"volume": "18",
"year": "2010"
},
{
"DOI": "10.1016/j.bmc.2016.05.013",
"article-title": "Identification, Synthesis and Evaluation of SARS-CoV and MERS-CoV 3C-like Protease Inhibitors",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "3035",
"journal-title": "Bioorg. Med. Chem.",
"key": "ref_80",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.1016/j.bioorg.2022.106255",
"doi-asserted-by": "crossref",
"key": "ref_81",
"unstructured": "Malebari, A.M., Ahmed, H.E.A., Ihmaid, S.K., Omar, A.M., Muhammad, Y.A., Althagfan, S.S., Aljuhani, N., El-Sayed, A.-A.A.A., Halawa, A.H., and El-Tahir, H.M. (2023). Exploring the Dual Effect of Novel 1,4-Diarylpyranopyrazoles as Antiviral and Anti-Inflammatory for the Management of SARS-CoV-2 and Associated Inflammatory Symptoms. Bioorg. Chem., 130."
},
{
"DOI": "10.3390/ph16030463",
"doi-asserted-by": "crossref",
"key": "ref_82",
"unstructured": "Musa, A., Abulkhair, H.S., Aljuhani, A., Rezki, N., Abdelgawad, M.A., Shalaby, K., El-Ghorab, A.H., and Aouad, M.R. (2023). Phenylpyrazolone-1,2,3-Triazole Hybrids as Potent Antiviral Agents with Promising SARS-CoV-2 Main Protease Inhibition Potential. Pharmaceuticals, 16."
},
{
"DOI": "10.1080/14756366.2024.2351861",
"article-title": "Exploring of N-Phthalimide-Linked 1,2,3-Triazole Analogues with Promising anti-SARS-CoV-2 Activity: Synthesis, Biological Screening, and Molecular Modelling Studies",
"author": "Aljuhani",
"doi-asserted-by": "crossref",
"first-page": "2351861",
"journal-title": "J. Enzyme Inhib. Med. Chem.",
"key": "ref_83",
"volume": "39",
"year": "2024"
},
{
"DOI": "10.1080/14756366.2019.1637861",
"article-title": "Novel Phthalimide Based Analogues: Design, Synthesis, Biological Evaluation, and Molecular Docking Studies",
"author": "Othman",
"doi-asserted-by": "crossref",
"first-page": "1259",
"journal-title": "J. Enzyme Inhib. Med. Chem.",
"key": "ref_84",
"volume": "34",
"year": "2019"
},
{
"DOI": "10.1016/j.molstruc.2022.134688",
"article-title": "Phthalimide/Naphthalimide Containing 1,2,3-Triazole Hybrids: Synthesis and Antimicrobial Evaluation",
"author": "Yadav",
"doi-asserted-by": "crossref",
"first-page": "134688",
"journal-title": "J. Mol. Struct.",
"key": "ref_85",
"volume": "1276",
"year": "2023"
},
{
"DOI": "10.1016/j.carres.2007.03.009",
"article-title": "Synthesis and Anti-Inflammatory Activity of N-Phthalimidomethyl 2,3-Dideoxy- and 2,3-Unsaturated Glycosides",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "1169",
"journal-title": "Carbohydr. Res.",
"key": "ref_86",
"volume": "342",
"year": "2007"
},
{
"DOI": "10.3390/life12091341",
"doi-asserted-by": "crossref",
"key": "ref_87",
"unstructured": "Al-Humaidi, J.Y., Shaaban, M.M., Rezki, N., Aouad, M.R., Zakaria, M., Jaremko, M., Hagar, M., and Elwakil, B.H. (2022). 1,2,3-Triazole-Benzofused Molecular Conjugates as Potential Antiviral Agents against SARS-CoV-2 Virus Variants. Life, 12."
},
{
"DOI": "10.1038/s41392-021-00619-y",
"article-title": "Ceftazidime Is a Potential Drug to Inhibit SARS-CoV-2 Infection in Vitro by Blocking Spike Protein–ACE2 Interaction",
"author": "Lin",
"doi-asserted-by": "crossref",
"first-page": "198",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_88",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1002/chem.202103861",
"article-title": "Anti-SARS-CoV-2 Inhibitory Profile of New Quinoline Compounds in Cell Culture-Based Infection Models",
"author": "Herrmann",
"doi-asserted-by": "crossref",
"first-page": "e202103861",
"journal-title": "Chem. Eur. J.",
"key": "ref_89",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/j.bioorg.2015.10.001",
"article-title": "Some New Indole–Coumarin Hybrids; Synthesis, Anticancer and Bcl-2 Docking Studies",
"author": "Kamath",
"doi-asserted-by": "crossref",
"first-page": "101",
"journal-title": "Bioorg. Chem.",
"key": "ref_90",
"volume": "63",
"year": "2015"
},
{
"DOI": "10.1021/acsomega.3c01085",
"article-title": "Novel Fluorescent Benzothiazolyl-Coumarin Hybrids as Anti-SARS-COVID-2 Agents Supported by Molecular Docking Studies: Design, Synthesis, X-Ray Crystal Structures, DFT, and TD-DFT/PCM Calculations",
"author": "Abdallah",
"doi-asserted-by": "crossref",
"first-page": "19587",
"journal-title": "ACS Omega",
"key": "ref_91",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1111/jphp.13331",
"article-title": "Benzothiazoles as Potential Antiviral Agents",
"author": "Asiri",
"doi-asserted-by": "crossref",
"first-page": "1459",
"journal-title": "J. Pharm. Pharmacol.",
"key": "ref_92",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1021/acsomega.3c10299",
"article-title": "Development in the Synthesis of Bioactive Thiazole-Based Heterocyclic Hybrids Utilizing Phenacyl Bromide",
"author": "Kumari",
"doi-asserted-by": "crossref",
"first-page": "18709",
"journal-title": "ACS Omega",
"key": "ref_93",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.1021/acsomega.3c01987",
"article-title": "Synthesis and Biological Evaluation of Benzothiazolyl-Pyridine Hybrids as New Antiviral Agents against H5N1 Bird Flu and SARS-COV-2 Viruses",
"author": "Metwally",
"doi-asserted-by": "crossref",
"first-page": "36636",
"journal-title": "ACS Omega",
"key": "ref_94",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1016/j.jiph.2020.09.002",
"article-title": "In Silico Molecular Docking: Evaluation of Coumarin Based Derivatives against SARS-CoV-2",
"author": "Chidambaram",
"doi-asserted-by": "crossref",
"first-page": "1671",
"journal-title": "J. Infect. Public Health",
"key": "ref_95",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1021/acsomega.3c02079",
"article-title": "Discovery of Hybrid Thiouracil–Coumarin Conjugates as Potential Novel Anti-SARS-CoV-2 Agents Targeting the Virus’s Polymerase “RdRp” as a Confirmed Interacting Biomolecule",
"author": "Vishwanath",
"doi-asserted-by": "crossref",
"first-page": "27056",
"journal-title": "ACS Omega",
"key": "ref_96",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1002/slct.202300605",
"article-title": "Synthesis and Virtual Screening of Pyrazolothiazole Conjugates as Promising SARS-CoV-2 Inhibitors",
"author": "Seliem",
"doi-asserted-by": "crossref",
"first-page": "e202300605",
"journal-title": "ChemistrySelect",
"key": "ref_97",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1016/j.bmcl.2022.129105",
"doi-asserted-by": "crossref",
"key": "ref_98",
"unstructured": "Hosseini Nasab, N., Azimian, F., Shim, R.S., Eom, Y.S., Shah, F.H., and Kim, S.J. (2023). Synthesis, Anticancer Evaluation, and Molecular Docking Studies of Thiazolyl-Pyrazoline Derivatives. Bioorg. Med. Chem. Lett., 80."
},
{
"DOI": "10.1016/j.ejmech.2021.113402",
"article-title": "4-4-(Anilinomethyl)-3-[4-(Trifluoromethyl)Phenyl]-1H-Pyrazol-1-Ylbenzoic Acid Derivatives as Potent Anti-Gram-Positive Bacterial Agents",
"author": "Hansa",
"doi-asserted-by": "crossref",
"first-page": "113402",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_99",
"volume": "219",
"year": "2021"
},
{
"DOI": "10.1002/ardp.201400083",
"article-title": "Novel Quinazolin-4(3H)-one/Schiff Base Hybrids as Antiproliferative and Phosphodiesterase 4 Inhibitors: Design, Synthesis, and Docking Studies",
"author": "Canzoneri",
"doi-asserted-by": "crossref",
"first-page": "650",
"journal-title": "Arch. Pharm.",
"key": "ref_100",
"volume": "347",
"year": "2014"
},
{
"DOI": "10.1039/D0RA06424F",
"article-title": "Quinazoline-Schiff Base Conjugates: In Silico Study and ADMET Predictions as Multi-Target Inhibitors of Coronavirus (SARS-CoV-2) Proteins",
"author": "Mansour",
"doi-asserted-by": "crossref",
"first-page": "34033",
"journal-title": "RSC Adv.",
"key": "ref_101",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.bmcl.2007.01.101",
"article-title": "Synthesis and Bioactivity of 4-Alkyl(Aryl)Thioquinazoline Derivatives",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "2193",
"journal-title": "Bioorg. Med. Chem. Lett.",
"key": "ref_102",
"volume": "17",
"year": "2007"
},
{
"DOI": "10.1016/j.ejmech.2020.112706",
"article-title": "Design, Synthesis and in Vitro Anti-Influenza A Virus Evaluation of Novel Quinazoline Derivatives Containing S-Acetamide and NH-Acetamide Moieties at C-4",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "112706",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_103",
"volume": "206",
"year": "2020"
},
{
"DOI": "10.3390/molecules27185923",
"doi-asserted-by": "crossref",
"key": "ref_104",
"unstructured": "Wyman, K.A., Girgis, A.S., Surapaneni, P.S., Moore, J.M., Abo Shama, N.M., Mahmoud, S.H., Mostafa, A., Barghash, R.F., Juan, Z., and Dobaria, R.D. (2022). Synthesis of Potential Antiviral Agents for SARS-CoV-2 Using Molecular Hybridization Approach. Molecules, 27."
},
{
"DOI": "10.1016/j.ejmech.2014.10.065",
"article-title": "A Review on Recent Developments of Indole-Containing Antiviral Agents",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "421",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_105",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1111/cbdd.14163",
"article-title": "Rhodanine Derivatives: An Insight into the Synthetic and Medicinal Perspectives as Antimicrobial and Antiviral Agents",
"author": "Chaurasyia",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Chem. Biol. Drug Des.",
"key": "ref_106",
"volume": "101",
"year": "2023"
},
{
"DOI": "10.1007/s11696-023-02804-5",
"article-title": "Fluorine-a Small Magic Bullet Atom in the Drug Development: Perspective to FDA Approved and COVID-19 Recommended Drugs",
"author": "Chandra",
"doi-asserted-by": "crossref",
"first-page": "4085",
"journal-title": "Chem. Pap.",
"key": "ref_107",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.3390/ph15030376",
"doi-asserted-by": "crossref",
"key": "ref_108",
"unstructured": "El-Kalyoubi, S.A., Ragab, A., Abu Ali, O.A., Ammar, Y.A., Seadawy, M.G., Ahmed, A., and Fayed, E.A. (2022). One-Pot Synthesis and Molecular Modeling Studies of New Bioactive Spiro-Oxindoles Based on Uracil Derivatives as SARS-CoV-2 Inhibitors Targeting RNA Polymerase and Spike Glycoprotein. Pharmaceuticals, 15."
},
{
"DOI": "10.1039/D3OB01122D",
"article-title": "Hybrid Molecules Based on an Emodin Scaffold. Synthesis and Activity against SARS-CoV-2 and Plasmodium",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "7382",
"journal-title": "Org. Biomol. Chem.",
"key": "ref_109",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1002/ptr.5631",
"article-title": "Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "1207",
"journal-title": "Phytother. Res.",
"key": "ref_110",
"volume": "30",
"year": "2016"
},
{
"DOI": "10.1038/s41598-021-97268-6",
"doi-asserted-by": "crossref",
"key": "ref_111",
"unstructured": "Hurmach, V.V., Platonov, M.O., Prylutska, S.V., Scharff, P., Prylutskyy, Y.I., and Ritter, U. (2021). C60 Fullerene against SARS-CoV-2 Coronavirus: An In Silico Insight. Sci. Rep., 11."
},
{
"DOI": "10.1002/ejoc.202201301",
"article-title": "Synthesis of [60]Fullerene Hybrids Endowed with Steroids and Monosaccharides: Theoretical Underpinning as Promising anti-SARS-CoV-2 Agents",
"author": "Lemos",
"doi-asserted-by": "crossref",
"first-page": "e202201301",
"journal-title": "Eur. J. Org. Chem.",
"key": "ref_112",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1016/j.ejmech.2024.116671",
"article-title": "Novel Compounds with Dual Inhibition Activity against SARS-CoV-2 Critical Enzymes RdRp and Human TMPRSS2",
"author": "Soliman",
"doi-asserted-by": "crossref",
"first-page": "116671",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_113",
"volume": "276",
"year": "2024"
},
{
"DOI": "10.1039/C5RA17920C",
"article-title": "Phenyl-Guanidine Derivatives as Potential Therapeutic Agents for Glioblastoma Multiforme: Catalytic Syntheses, Cytotoxic Effects and DNA Affinity",
"author": "Bravo",
"doi-asserted-by": "crossref",
"first-page": "8267",
"journal-title": "RSC Adv.",
"key": "ref_114",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1038/nrd4010",
"article-title": "Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases",
"author": "Jordheim",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_115",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1038/s41392-023-01482-9",
"article-title": "Discovery of Novel Non-Peptidic and Non-Covalent Small-Molecule 3CLpro Inhibitors as Potential Candidate for COVID-19 Treatment",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_116",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.4c00739",
"article-title": "Discovery of Novel Nonpeptidic and Noncovalent Small Molecule 3CL pro Inhibitors as Anti-SARS-CoV-2 Drug Candidate",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "12760",
"journal-title": "J. Med. Chem.",
"key": "ref_117",
"volume": "67",
"year": "2024"
},
{
"DOI": "10.1016/j.bmc.2022.116674",
"doi-asserted-by": "crossref",
"key": "ref_118",
"unstructured": "Sharma, V., Das, R., Kumar Mehta, D., Gupta, S., Venugopala, K.N., Mailavaram, R., Nair, A.B., Shakya, A.K., and Kishore Deb, P. (2022). Recent Insight into the Biological Activities and SAR of Quinolone Derivatives as Multifunctional Scaffold. Bioorg. Med. Chem., 59."
}
],
"reference-count": 118,
"references-count": 118,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1420-3049/29/22/5403"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Insights into SARS-CoV-2: Small-Molecule Hybrids for COVID-19 Treatment",
"type": "journal-article",
"volume": "29"
}