A Randomized Trial of Nafamostat for Covid-19
et al., NEJM Evidence, doi:10.1056/EVIDoa2300132, ASCOT, NCT04483960, Oct 2023
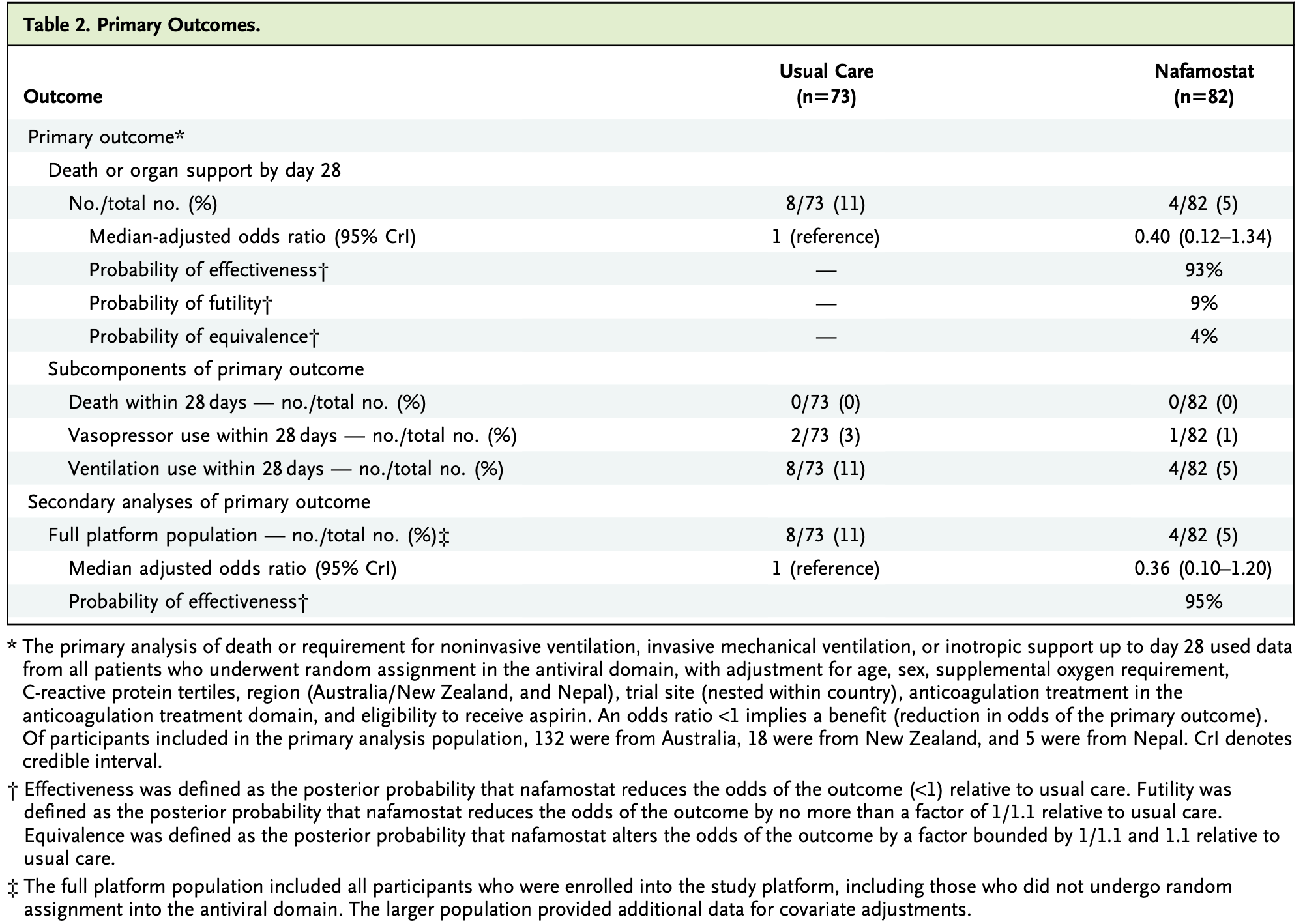
RCT 160 hospitalized non-critically ill COVID-19 patients showing a 93% posterior probability that nafamostat reduced the odds of death or receipt of ventilatory or vasopressor support by day 28 compared to usual care. Nafamostat, a TMPRSS2 inhibitor with potent in vitro antiviral activity against SARS-CoV-2, was administered as a continuous intravenous infusion for up to 7 days. The trial was conducted across 21 hospitals in Australia, New Zealand, and Nepal. Despite promising results, the trial was stopped early due to slowing recruitment, low event rates, and funding constraints, limiting definitive conclusions. Authors note that the posterior probability of effectiveness was higher among those with earlier disease onset, but lower during the Omicron era when variants were less dependent on the TMPRSS2 pathway.
Study covers TMPRSS2 inhibitors and nafamostat.
|
risk of mechanical ventilation, 55.5% lower, RR 0.45, p = 0.23, treatment 4 of 82 (4.9%), control 8 of 73 (11.0%), NNT 16, day 28.
|
|
risk of progression, 57.2% lower, RR 0.43, p = 0.14, treatment 4 of 82 (4.9%), control 8 of 73 (11.0%), NNT 16, adjusted per study, odds ratio converted to relative risk, death, ventilation, or vasopressor support, day 28.
|
|
risk of no recovery, 28.0% lower, OR 0.72, p = 0.36, treatment 82, control 73, WHO scale, day 28, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Morpeth et al., 24 Oct 2023, Randomized Controlled Trial, multiple countries, peer-reviewed, 64 authors, study period 17 May, 2021 - 12 August, 2022, trial NCT04483960 (history) (ASCOT).
Contact: steven.tong@unimelb.edu.au.
A Randomized Trial of Nafamostat for Covid-19
NEJM Evidence, doi:10.1056/evidoa2300132
BACKGROUND Nafamostat mesylate is a potent in vitro antiviral agent that inhibits the host transmembrane protease serine 2 enzyme used by severe acute respiratory syndrome coronavirus 2 for cell entry. METHODS This open-label, pragmatic, randomized clinical trial in Australia, New Zealand, and Nepal included noncritically ill hospitalized patients with coronavirus disease 2019 (Covid-19). Participants were randomly assigned to usual care or usual care plus nafamostat. The primary end point was death (any cause) or receipt of new invasive or noninvasive ventilation or vasopressor support within 28 days after randomization. Analysis was with a Bayesian logistic model in which an adjusted odds ratio <1.0 indicates improved outcomes with nafamostat. Enrollment was closed due to falling numbers of eligible patients.
RESULTS We screened 647 patients in 21 hospitals (15 in Australia, 4 in New Zealand, and 2 in Nepal) and enrolled 160 participants from May 2021 to August 2022. In the intention-to-treat population, the primary end point occurred in 8 (11%) of 73 patients with usual care and 4 (5%) of 82 with nafamostat. The median adjusted odds ratio for the primary end point for nafamostat was 0.40 (95% credible interval, 0.12 to 1.34) with a posterior probability of effectiveness (adjusted odds ratio <1.0) of 93%. For usual care
The funding bodies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Author disclosures and other supplementary materials are available at evidence.nejm.org. The data-sharing statement is provided in the Supplementary Appendix. cine, Nîmes University Hospital at The University of Montpellier, Nîmes, France
References
Aggarwal, Akerman, Milogiannakis, SARS-CoV-2 Omicron BA.5: evolving tropism and evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern, EBioMedicine, doi:10.1016/j.ebiom.2022.104270
Choi, Kang, Jang, Nafamostat mesilate as an anticoagulant during continuous renal replacement therapy in patients with high bleeding risk: a randomized clinical trial, Medicine (Baltimore), doi:10.1097/MD.0000000000002392
Denholm, Venkatesh, Davis, ASCOT ADAPT study of Covid-19 therapeutics in hospitalised patients: an international multicentre adaptive platform trial, Trials, doi:10.1186/s13063-022-06929-y
Hatesuer, Bertram, Mehnert, TMPRSS2 is essential for influenza H1N1 virus pathogenesis in mice, PLoS Pathog, doi:10.1371/journal.ppat.1003774
Hempel, Raich, Olsson, Molecular mechanism of inhibiting the SARS-CoV-2 cell entry facilitator TMPRSS2 with camostat and nafamostat, Chem Sci (Camb), doi:10.1039/D0SC05064D
Hirota, Shimosegawa, Kitamura, Continuous regional arterial infusion versus intravenous administration of the protease inhibitor nafamostat mesilate for predicted severe acute pancreatitis: a multicenter, randomized, open-label, phase 2 trial, J Gastroenterol, doi:10.1007/s00535-019-01644-z
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hoffmann, Schroeder, Kleine-Weber, Ma, Drosten et al., Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for Covid-19, Antimicrob Agents Chemother, doi:10.1128/AAC.00754-20
Ikeda, Okugawa, Kashiwabara, Multicenter, singleblind, randomized controlled study of the efficacy and safety of favipiravir and nafamostat mesilate in patients with Covid-19 pneumonia, Int J Infect Dis, doi:10.1016/j.ijid.2022.12.039
Kastenhuber, Mercadante, Nilsson-Payant, Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry, eLife, doi:10.7554/eLife.77444
Ko, Jeon, Ryu, Kim, Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells, J Med Virol, doi:10.1002/jmv.26397
Kodama, Imai, Asai, Incidence and risk factors for hyperkalaemia in patients treated for Covid-19 with nafamostat mesylate, J Clin Pharm Ther, doi:10.1111/jcpt.13646
Lee, Murthy, Corpo, Remdesivir for the treatment of Covid-19: a systematic review and meta-analysis, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.04.018
Li, Meyerholz, Bartlett, Mccray, The TMPRSS2 inhibitor nafamostat reduces SARS-CoV-2 pulmonary infection in mouse models of Covid-19, mBio, doi:10.1128/mBio.00970-21
Mantzourani, Vasilakaki, Gerogianni, Kokotos, The discovery and development of transmembrane serine protease 2 (TMPRSS2) inhibitors as candidate drugs for the treatment of Covid-19, Expert Opin Drug Discov, doi:10.1080/17460441.2022.2029843
Mcfadyen, Stevens, Peter, The emerging threat of (micro)thrombosis in Covid-19 and its therapeutic implications, Circ Res, doi:10.1161/CIRCRESAHA.120.317447
Mcquilten, Venkatesh, Jha, Anticoagulation strategies in non-critically ill patients with Covid-19, NEJM Evid, doi:10.1056/EVIDoa2200293
Meng, Abdullahi, Ferreira, Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity, Nature, doi:10.1038/s41586-022-04474-x
Muto, Imai, Asano, Mechanisms of hyperkalemia caused by nafamostat mesilate, Gen Pharmacol, doi:10.1016/0306-3623(95)00072-0
Oka, Homma, Nishino, Retroperitoneal hemorrhage in patients with Covid-19 undergoing hemodialysis: three case reports, Intern Med, doi:10.2169/internalmedicine.8976-21
Okajima, Takahashi, Kaji, Ogawa, Mouri, Nafamostat mesylate-induced hyperkalemia in critically ill patients with Covid-19: four case reports, World J Clin Cases, doi:10.12998/wjcc.v8.i21.5320
Ookawara, Tabei, Sakurai, Sakairi, Furuya et al., Additional mechanisms of nafamostat mesilate-associated hyperkalaemia, Eur J Clin Pharmacol, doi:10.1007/s002280050176
Quinn, Gaughan, Bruce, Randomised controlled trial of intravenous nafamostat mesylate in Covid pneumonitis: phase 1b/2a experimental study to investigate safety, pharmacokinetics and pharmacodynamics, EBioMedicine, doi:10.1016/j.ebiom.2022.103856
Sakai, Ami, Tahara, The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses, J Virol, doi:10.1128/JVI.03677-13
Soma, Fujii, Yoshifuji, Nafamostat mesylate monotherapy in patients with moderate Covid-19: a single-center, retrospective study, Jpn J Infect Dis, doi:10.7883/yoken.JJID.2021.699
Yamada, Asakura, Therapeutic strategies for disseminated intravascular coagulation associated with aortic aneurysm, Int J Mol Sci, doi:10.3390/ijms23031296
Yamamoto, Kiso, Sakai-Tagawa, The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-typedependent manner, Viruses, doi:10.3390/v12060629
Yoshioka, Daizumoto, Tada, Retroperitoneal hemorrhage in a patient with coronavirus disease 2019 (Covid-19): a case report, J Med Invest, doi:10.2152/jmi.69.148
Zhuravel, Khmelnitskiy, Burlaka, Nafamostat in hospitalized patients with moderate to severe Covid-19 pneumonia: a randomised phase II clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101169
DOI record:
{
"DOI": "10.1056/evidoa2300132",
"ISSN": [
"2766-5526"
],
"URL": "http://dx.doi.org/10.1056/EVIDoa2300132",
"alternative-id": [
"10.1056/EVIDoa2300132"
],
"author": [
{
"affiliation": [
{
"name": "Department of Microbiology and Infectious Diseases, Middlemore Hospital, Te Whatu Ora Counties Makukau, Auckland, New Zealand"
},
{
"name": "Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand"
}
],
"family": "Morpeth",
"given": "Susan C.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Intensive Care Medicine, The University of Queensland at Princess Alexandra Hospital, Woolloongabba, QLD, Australia"
},
{
"name": "Department of Intensive Care Medicine, The University of Queensland at The Wesley Hospital, Toowong, QLD, Australia"
},
{
"name": "The George Institute for Global Health, Newtown, NSW, Australia"
}
],
"family": "Venkatesh",
"given": "Balasubramanian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine and Health, The University of Sydney School of Public Health, Sydney"
}
],
"family": "Totterdell",
"given": "James A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "McPhee",
"given": "Grace M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia"
},
{
"name": "Clinical Epidemiology and Biostatistics Unit, Murdoch Children’s Research Institute, Melbourne, VIC, Australia"
}
],
"family": "Mahar",
"given": "Robert K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Health and Clinical Analytics, The University of Sydney School of Public Health, Sydney"
}
],
"family": "Jones",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Bandara",
"given": "Methma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Barina",
"given": "Lauren A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Bir Hospital, Kathmandu, Nepal"
},
{
"name": "Department of Infectious Diseases, Perth Children’s Hospital, Perth, WA, Australia"
}
],
"family": "Basnet",
"given": "Bhupendra K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Nedlands, WA, Australia"
}
],
"family": "Bowen",
"given": "Asha C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, The University of Queensland, Herston, QLD, Australia"
},
{
"name": "Department of Infectious Diseases, Prince Charles Hospital, Merthyr Tydfil, United Kingdom"
}
],
"family": "Burke",
"given": "Andrew J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Sleep Medicine, Campbelltown Hospital, Campbelltown, NSW, Australia"
},
{
"name": "Western Sydney University School of Medicine, Campbelltown, NSW, Australia"
}
],
"family": "Cochrane",
"given": "Belinda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
},
{
"name": "Victorian Infectious Diseases Service, The Royal Melbourne Hospital, Melbourne, VIC, Australia"
}
],
"family": "Denholm",
"given": "Justin T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, National Academy of Medical Sciences at Bir Hospital, Kathmandu, Nepal"
}
],
"family": "Dhungana",
"given": "Ashesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Viral Hepatitis Clinical Research Program, Kirby Institute, University of New South Wales, Kensington, NSW, Australia"
},
{
"name": "Department of Infectious Diseases, St. Vincent’s Hospital, Melbourne, VIC, Australia"
}
],
"family": "Dore",
"given": "Gregory J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Blacktown Hospital, Blacktown, NSW, Australia"
}
],
"family": "Dotel",
"given": "Ravindra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Te Whatu Ora Health New Zealand at Auckland City Hospital, Auckland, New Zealand"
},
{
"name": "School of Pharmacy, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand"
}
],
"family": "Duffy",
"given": "Eamon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Otago, Dunedin, New Zealand"
},
{
"name": "Respiratory Services, Dunedin Hospital, Dunedin, New Zealand"
}
],
"family": "Dummer",
"given": "Jack",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Infectious Diseases, NSW Health Pathology Liverpool, Liverpool, NSW, Australia"
}
],
"family": "Foo",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Infectious Diseases, Wagga Wagga Base Hospital, Wagga Wagga, Australia"
}
],
"family": "Gilbey",
"given": "Timothy L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Program, The George Institute for Global Health, New Town, NSW, Australia"
},
{
"name": "Critical Care Program, The University of New South Wales, Sydney"
},
{
"name": "Malcolm Fisher Department of Intensive Care, Royal North Shore Hospital, St. Leonards, NSW, Australia"
}
],
"family": "Hammond",
"given": "Naomi E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Infectious Diseases, NSW Health Pathology, St. Leonards, St. Leonards, NSW, Australia"
}
],
"family": "Hudson",
"given": "Bernard J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The George Institute for Global Health, Newtown, NSW, Australia"
}
],
"family": "Jha",
"given": "Vivekanand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, The George Institute for Global Health, Pune, Maharashta, India"
}
],
"family": "Jevaji",
"given": "Purnima R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, The George Institute for Global Health, Vellore, India"
},
{
"name": "Prasanna School of Public Health, Manipal Academy of Higher Education, Karnataka, India"
}
],
"family": "John",
"given": "Oommen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, The George Institute for Global Health, Pune, Maharashta, India"
}
],
"family": "Joshi",
"given": "Rajesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Wellcome Trust Research Laboratory, Chartered Accountants Australia and New Zealand, Sydney"
}
],
"family": "Kang",
"given": "Gagandeep",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Critical Care Program, The George Institute for Global Health, New Town, NSW, Australia"
}
],
"family": "Kaur",
"given": "Baldeep",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Zoonotic Virus Laboratory, Institut Pasteur Korea, Bundang-gu, Gyeonggi-do, Republic of Korea"
}
],
"family": "Kim",
"given": "Seungtaek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maharajgunj Medical Campus, Institute of Medicine, Maharajgunj, Nepal"
}
],
"family": "Das",
"given": "Santa Kumar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Eastern Health, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Lau",
"given": "Jillian S.Y.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "UQ Centre for Clinical Research, Faculty of Medicine, The University of Queensland, Queensland, QLD, Australia"
}
],
"family": "Littleford",
"given": "Roberta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Nedlands, WA, Australia"
},
{
"name": "Centre for Child Health Research, University of Western Australia Medical School, Nedlands, WA, Australia"
}
],
"family": "Marsh",
"given": "Julie A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NHMRC Clinical Trials Centre, Faculty of Medicine and Health, The University of Sydney, Sydney"
}
],
"family": "Marschner",
"given": "Ian C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, St. Vincent’s Hospital Sydney, Sydney"
},
{
"name": "Therapeutic and Vaccine Research Program, The Kirby Institute at The University of New South Wales, Kensington, NSW, Australia"
}
],
"family": "Matthews",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Otago Christchurch, Christchurch, New England"
}
],
"family": "Maze",
"given": "Michael J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Care Medicine, Te Whatu Ora — Health New Zealand, Wellington, New Zealand"
}
],
"family": "McArthur",
"given": "Colin J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Haematology, Alfred Hospital, Melbourne, VIC, Australia"
},
{
"name": "Atherothrombosis and Vascular Biology Program, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia"
}
],
"family": "McFadyen",
"given": "James D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Alfred Health and Monash University, Melbourne, VIC, Australia"
}
],
"family": "McMahon",
"given": "James H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Haematology, Monash Health, Melbourne, VIC, Australia"
},
{
"name": "School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia"
}
],
"family": "McQuilten",
"given": "Zoe K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Western Health, Footscray, VIC, Australia"
}
],
"family": "Molton",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Mora",
"given": "Jocelyn M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Research, The George Institute for Global Health, Pune, Maharashta, India"
}
],
"family": "Mudaliar",
"given": "Vijaybabu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Nguyen",
"given": "Vi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Infectious Diseases, NSW Health Pathology Westmead Hospital, Newcastle, NSW, Australia"
},
{
"name": "Faculty of Medicine and Health, University of Sydney Westmead Clinical School, Sydney"
}
],
"family": "O’Sullivan",
"given": "Matthew V.N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Maharajgunj Medical Campus, Institute of Medicine, Maharajgunj, Nepal"
}
],
"family": "Pant",
"given": "Suman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Business Development Team, Chong Kun Dang Pharmaceutical Corp., Dongbaekjukjeon-daero, Giheung-gu Yongin, Kyeonggi-do, Republic of Korea"
}
],
"family": "Park",
"given": "Jaha E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Saw Swee Hock School of Public Health, National Institute of Singapore, Singapore"
}
],
"family": "Paterson",
"given": "David L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
},
{
"name": "Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Price",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Te Whatu Ora Health New Zealand Capital, Coast and Hutt Valley, Wellington, New Zealand"
},
{
"name": "Department of Medicine, Wellington School of Medicine, University of Otago, Wellington, New Zealand"
}
],
"family": "Raymond",
"given": "Nigel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Sleep Medicine, The Royal Melbourne Hospital, Melbourne, VIC, Australia"
}
],
"family": "Rees",
"given": "Megan A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Royal Perth Hospital, Perth, WA, Australia"
},
{
"name": "Department of Microbiology, PathWest Laboratory Medicine, Nedlands, WA, Australia"
}
],
"family": "Robinson",
"given": "James O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Inflammatory Diseases, School of Clinical Sciences, Monash University, Clayton, VIC, Australia"
},
{
"name": "Department of Infectious Diseases, Monash Health, Clayton, VIC, Australia"
}
],
"family": "Rogers",
"given": "Benjamin A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Virology, Institute Pasteur Korea, Bundang-gu, Gyeonggi-do, Republic of Korea"
}
],
"family": "Ryu",
"given": "Wang-Shick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Victorian Infectious Diseases Service, The Royal Melbourne Hospital, Melbourne, VIC, Australia"
}
],
"family": "Sasadeusz",
"given": "Joe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Wollongong Hospital, Kingoonya, NSW, Australia"
},
{
"name": "Graduate School of Medicine, University of Wollongong, Wollonngong, NSW, Australia"
}
],
"family": "Shum",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine and Health, The University of Sydney School of Public Health, Sydney"
},
{
"name": "Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Nedlands, WA, Australia"
}
],
"family": "Snelling",
"given": "Thomas L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
}
],
"family": "Sommerville",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chartered Accountants Australia and New Zealand, Sydney"
}
],
"family": "Trask",
"given": "Nanette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
},
{
"name": "Victorian Infectious Diseases Service, The Royal Melbourne Hospital, Melbourne, VIC, Australia"
},
{
"name": "Department of Infectious Diseases, Alfred Health and Monash University, Melbourne, VIC, Australia"
}
],
"family": "Lewin",
"given": "Sharon R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Research, Medical Research Institute of New Zealand, Wellington, New Zealand"
},
{
"name": "Department of Infectious Diseases, Auckland City Hospital, Auckland, New Zealand"
}
],
"family": "Hills",
"given": "Thomas E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine and Public Health, The University of Newcastle, New Castle, Australia"
},
{
"name": "Global and Tropical Health Division, Menzies School of Health Research, Darwin, NT, Australia"
}
],
"family": "Davis",
"given": "Joshua S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, The University of Queensland Centre for Clinical Research, Herston, QLD, Australia"
},
{
"name": "Metro North Health, Herston Infectious Diseases Institute, Herston, QLD, Australia"
},
{
"name": "Departments of Pharmacy and Intensive Care Medicine, Royal Brisbane and Women’s Hospital, Herston, QLD, Australia"
},
{
"name": "Division of Anaesthesiology, Critical Care Emergency and Pain Medicine, Nîmes University Hospital at The University of Montpellier, Nîmes, France"
}
],
"family": "Roberts",
"given": "Jason A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Melbourne, VIC, Australia"
},
{
"name": "Victorian Infectious Diseases Service, The Royal Melbourne Hospital, Melbourne, VIC, Australia"
}
],
"family": "Tong",
"given": "Steven Y.C.",
"sequence": "additional"
}
],
"container-title": "NEJM Evidence",
"container-title-short": "NEJM Evidence",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
18
]
],
"date-time": "2023-10-18T17:00:32Z",
"timestamp": 1697648432000
},
"deposited": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T21:29:14Z",
"timestamp": 1698960554000
},
"indexed": {
"date-parts": [
[
2025,
4,
10
]
],
"date-time": "2025-04-10T08:11:20Z",
"timestamp": 1744272680097
},
"is-referenced-by-count": 6,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
10,
24
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
10,
24
]
]
}
},
"language": "en",
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2023,
10,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
10,
24
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_2_2"
},
{
"DOI": "10.1080/17460441.2022.2029843",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_3_2"
},
{
"DOI": "10.1128/AAC.00754-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_4_2"
},
{
"DOI": "10.1039/D0SC05064D",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_5_2"
},
{
"DOI": "10.3390/v12060629",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_6_2"
},
{
"DOI": "10.7554/eLife.77444",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_7_2"
},
{
"DOI": "10.1128/mBio.00970-21",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_8_2"
},
{
"DOI": "10.1007/s00535-019-01644-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_9_2"
},
{
"DOI": "10.3390/ijms23031296",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_10_2"
},
{
"DOI": "10.1097/MD.0000000000002392",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_11_2"
},
{
"DOI": "10.1002/jmv.26397",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_12_2"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317447",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_13_2"
},
{
"DOI": "10.1016/j.ebiom.2022.103856",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_14_2"
},
{
"DOI": "10.1016/j.ijid.2022.12.039",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_15_2"
},
{
"DOI": "10.1016/j.eclinm.2021.101169",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_16_2"
},
{
"DOI": "10.1186/s13063-022-06929-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_17_2"
},
{
"DOI": "10.1056/EVIDoa2200293",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_18_2"
},
{
"DOI": "10.1016/j.cmi.2022.04.018",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_19_2"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_20_2"
},
{
"DOI": "10.1016/j.ebiom.2022.104270",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_21_2"
},
{
"DOI": "10.7883/yoken.JJID.2021.699",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_22_2"
},
{
"DOI": "10.1111/jcpt.13646",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_23_2"
},
{
"DOI": "10.12998/wjcc.v8.i21.5320",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_24_2"
},
{
"DOI": "10.1016/0306-3623(95)00072-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_25_2"
},
{
"DOI": "10.1007/s002280050176",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_26_2"
},
{
"DOI": "10.2169/internalmedicine.8976-21",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_27_2"
},
{
"DOI": "10.2152/jmi.69.148",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_28_2"
},
{
"DOI": "10.1128/JVI.03677-13",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_29_2"
},
{
"DOI": "10.1371/journal.ppat.1003774",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_30_2"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://evidence.nejm.org/doi/10.1056/EVIDoa2300132"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Randomized Trial of Nafamostat for Covid-19",
"type": "journal-article",
"volume": "2"
}
morpeth