Hydroxychloroquine for treatment of non-hospitalized adults with COVID-19: A meta-analysis of individual participant data of randomized trials
et al., Clinical and Translational Science, doi:10.1111/cts.13468, NCT04304053, Jan 2023
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 423 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
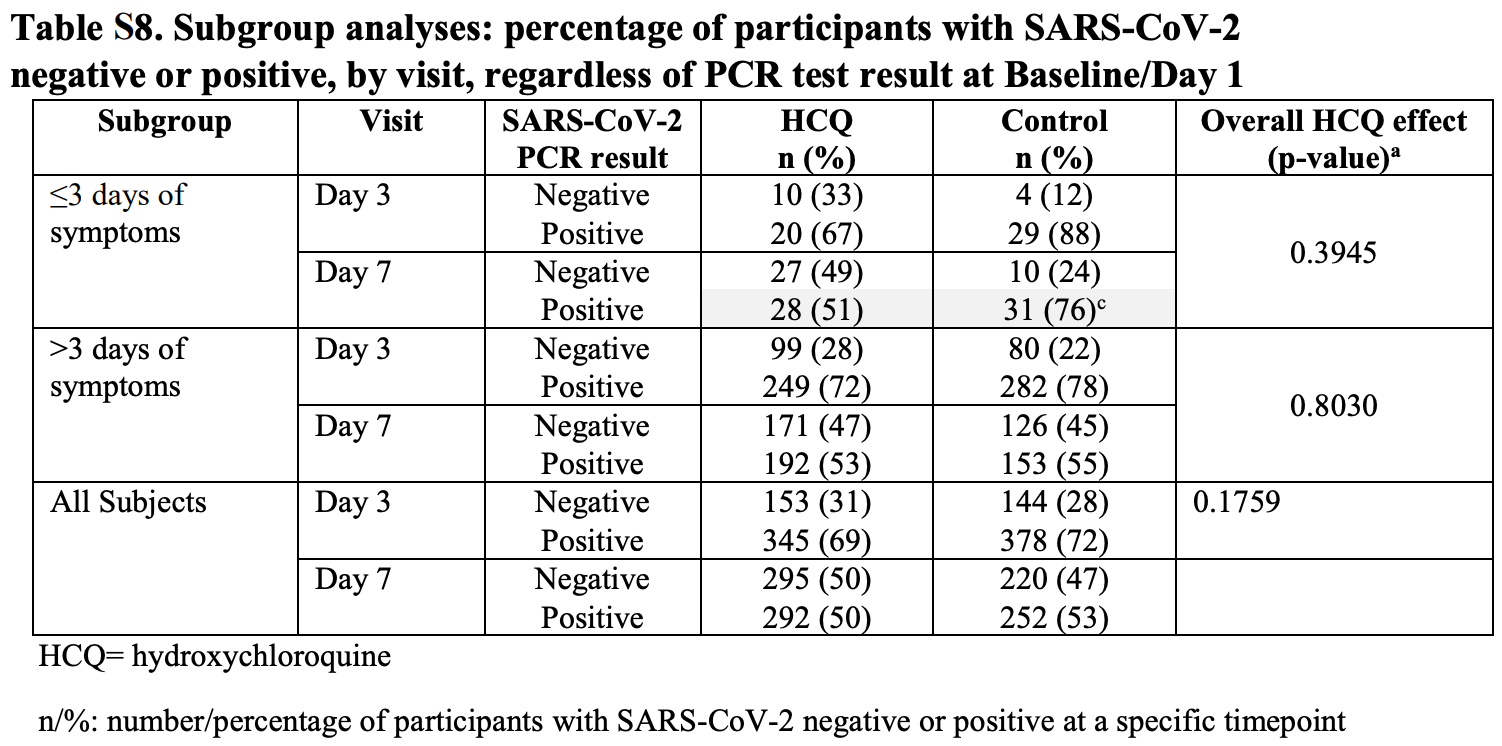
Extremely high COI (includes authors of trials playing a key role in the suppression of treatment, and funded by the Gates Foundation) IPD meta analysis of 11 HCQ outpatient treatment and prophylaxis trials, showing significantly improved viral clearance for treatment <4 days. This result is hidden in the supplementary material, and authors combine late treatment patients to generate a null result overall. Less than 20% of patients included had symptoms <4 days.
Hospitalization results for <4 days are not currently available, only combined results showing no significant difference. Early treatment hospitalization is the most important outcome analyzed in this study. We are unaware of a reason to leave these results out, other than to hide the efficacy of early treatment. For all current trials, early treatment shows 41% [28‑51%] lower hospitalization vs. 2% [-10‑16%] higher hospitalization for late treatment.
No individual study outcome results are provided, preventing error checking.
Authors state that "Individual data from participants who received HCQ combined with other drugs were excluded from the analysis", however this appears to have been selectively enforced - authors include patients treated with HCQ+azithromycin.
Authors indicate excluding 89 patients treated with darunavir for NCT04304053 (history), however the relevant paper1 does not show any patients receiving darunavir.
Authors state that "most [patients] were greater than or equal to 3 days from symptom onset (50-54%)", which appears to be incorrect in two ways. Table 2 shows that the cutoff was ≤3 and >3, i.e. ≥4 assuming only integer delays, and the percentages in the late treatment group range from 80-86% (of the patients where the delay is known).
10 meta-analyses show significant improvements with hydroxychloroquine for mortality2-5,
hospitalization2,
recovery6,
combined death/hospitalization/cases7,
cases8-10, and
viral clearance11.
Currently there are 37 HCQ for COVID-19 early treatment studies, showing 76% lower mortality [61‑85%], 67% lower ventilation [-710‑99%], 31% lower ICU admission [1‑53%], and 41% lower hospitalization [28‑51%].
|
risk of no viral clearance, 32.7% lower, RR 0.67, p = 0.02, treatment 28 of 55 (50.9%), control 31 of 41 (75.6%), NNT 4.0, <4 days symptoms, day 7, Table S8.
|
|
risk of no viral clearance, 24.1% lower, RR 0.76, p = 0.07, treatment 20 of 30 (66.7%), control 29 of 33 (87.9%), NNT 4.7, <4 days symptoms, day 3, Table S8.
|
|
risk of no viral clearance, 30.4% lower, RR 0.70, p = 0.02, treatment 26 of 47 (55.3%), control 31 of 39 (79.5%), NNT 4.1, <4 days symptoms, baseline positive, day 7, Table S9.
|
|
risk of no viral clearance, 12.5% lower, RR 0.87, p = 0.22, treatment 18 of 22 (81.8%), control 29 of 31 (93.5%), NNT 8.5, <4 days symptoms, baseline positive, day 3, Table S9.
|
|
risk of no viral clearance, 6.8% lower, RR 0.93, p = 0.24, treatment 292 of 587 (49.7%), control 252 of 472 (53.4%), NNT 27, including late treatment, day 7, Table S8.
|
|
risk of no viral clearance, 4.3% lower, RR 0.96, p = 0.30, treatment 345 of 498 (69.3%), control 378 of 522 (72.4%), NNT 32, including late treatment, day 3, Table S8.
|
|
risk of no viral clearance, 6.4% lower, RR 0.94, p = 0.27, treatment 283 of 532 (53.2%), control 250 of 440 (56.8%), NNT 28, including late treatment, baseline positive, day 7, Table S9.
|
|
risk of no viral clearance, 0.7% lower, RR 0.99, p = 0.88, treatment 334 of 440 (75.9%), control 374 of 489 (76.5%), NNT 174, including late treatment, baseline positive, day 3, Table S9.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Mitjà et al., Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial, Clinical Infectious Diseases, ciaa1009, doi:10.1093/cid/ciaa1009.
2.
Landsteiner de Sampaio Amêndola et al., COVID-19 Infection in Rheumatic Patients on Chronic Antimalarial Drugs: A Systematic Review and Meta-Analysis, Journal of Clinical Medicine, doi:10.3390/jcm11226865.
3.
Risch, H., Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis, American Journal of Epidemiology, kwaa093, 27 May 2020, doi:10.1093/aje/kwaa093.
4.
Risch (B), H., Response to: “Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients” and “Re: Early Outpatient Treatment of Symptomatic, High-Risk Covid-19 Patients that Should be Ramped-Up Immediately as Key to the Pandemic Crisis”, American Journal of Epidemiology, July 20, 2020, doi:10.1093/aje/kwaa152.
5.
Stricker et al., Hydroxychloroquine Pre-Exposure Prophylaxis for COVID-19 in Healthcare Workers from India: A Meta-Analysis, Journal of Infection and Public Health, doi:10.1016/j.jiph.2021.08.001.
6.
Prodromos et al., Hydroxychloroquine is effective, and consistently so used early, for Covid-19: A systematic review, New Microbes and New Infections, doi:10.1016/j.nmni.2020.100776.
7.
Ladapo et al., Randomized Controlled Trials of Early Ambulatory Hydroxychloroquine in the Prevention of COVID-19 Infection, Hospitalization, and Death: Meta-Analysis, medRxiv, doi:10.1101/2020.09.30.20204693.
8.
García-Albéniz et al., Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19, European Journal of Epidemiology, doi:10.1007/s10654-022-00891-4.
9.
Han et al., The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis and clinical assessment: an updated meta-analysis of randomized trials, Journal of Thoracic Disease, doi:10.21037/jtd-23-1043.
Mitjà et al., 4 Jan 2023, peer-reviewed, 27 authors, trial NCT04304053 (history).
Contact: omitja@lluita.org.
Abstract: DOI: 10.1111/cts.13468
| Revised: 18 November 2022 | Accepted: 22 November 2022
ARTICLE
Hydroxychloroquine for treatment of non-hospitalized
adults with COVID-19: A meta-analysis of individual
participant data of randomized trials
Oriol Mitjà1,2,3 | Gilmar Reis4,5 | David R. Boulware6 | Adam M. Spivak7 |
Ammar Sarwar8 | Christine Johnston9 | Brandon Webb10 | Michael D. Hill11 |
Davey Smith12 | Peter Kremsner13,14 | Marla Curran15 | David Carter16 |
Jim Alexander15 | Marc Corbacho1
| Todd C. Lee6 | Katherine Huppler Hullsiek6
Emily G. McDonald17 | Rachel Hess7
| Michael Hughes8 | Jared M. Baeten9 |
Ilan Schwartz18 | Luanne Metz11 | Lawrence Richer18 | Kara W. Chew19 |
Eric Daar20 | David Wohl21 | Michael Dunne15
|
1
Fight AIDS and Infectious Diseases Foundation, Barcelona, Spain
2
Hospital Universitari Germans Trias i Pujol, Badalona, Spain
3
Lihir Medical Center–International SOS, Lihir Island, Papua New Guinea
4
Research Division, Cardresearch Cardiologia Assistencial e de Pesquisa, Pontifícia Universidade Católica de Minas Gerais, Bello Horizonte, Brazil
5
Cytel Inc., Vancouver, British Columbia, Canada
6
Division of Infectious Diseases and International Medicine, Department of Medicine, University of Minnesota, Minneapolis, Minnesota, USA
7
University of Utah, Salt Lake City, Utah, USA
8
Harvard Medical School, Boston, Massachusetts, USA
9
Department of Medicine and Laboratory Medicine and Pathology, University of Washington, Seattle, Washington, USA
10
Intermountain Health Care, University of Utah, Salt Lake City, Utah, USA
11
University of Calgary, Calgary, Alberta, Canada
12
Division of Infectious Diseases & Global Public Health, UC San Diego School of Medicine, San Diego, California, USA
13
University Hospital of Tübingen, Tübingen, Germany
14
Centre de Recherches Médicales de Lambaréné, Lambaréné, Gabon
15
Bill & Melinda Gates Medical Research Institute, Cambridge, Massachusetts, USA
16
MMS Holdings, Canton, Michigan, USA
17
Division of General Internal Medicine, McGill University Health Center, Montreal, Quebec, Canada
18
University of Alberta, Edmonton, Canada
19
Division of Infectious Diseases, Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles, California, USA
20
Lundquist Institute at Harbor-UCLA Medical Center, Torrance, California, USA
21
School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
Correspondence
Oriol Mitjà, Fight AIDS and Infectious Diseases Foundation, Barcelona, Spain, Ctra. de Canyet s/n • Hosp. Univ. Germans Trias i Pujol, 08916
Badalona, Barcelona, Spain.
Email: omitja@lluita.org
This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any
medium, provided the original work is properly cited and is not used for commercial purposes.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Clin Transl Sci. 2023;00:1–12.
www.cts-journal.com
| 1
17528062, 0,
DOI record:
{
"DOI": "10.1111/cts.13468",
"ISSN": [
"1752-8054",
"1752-8062"
],
"URL": "http://dx.doi.org/10.1111/cts.13468",
"alternative-id": [
"10.1111/cts.13468"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-08-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-11-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-01-04"
}
],
"author": [
{
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation Barcelona Spain"
},
{
"name": "Hospital Universitari Germans Trias i Pujol Badalona Spain"
},
{
"name": "Lihir Medical Center–International SOS Lihir Island, Papua New Guinea"
}
],
"family": "Mitjà",
"given": "Oriol",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Research Division, Cardresearch Cardiologia Assistencial e de Pesquisa Pontifícia Universidade Católica de Minas Gerais Bello Horizonte Brazil"
},
{
"name": "Cytel Inc. Vancouver British Columbia Canada"
}
],
"family": "Reis",
"given": "Gilmar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, Department of Medicine University of Minnesota Minneapolis Minnesota USA"
}
],
"family": "Boulware",
"given": "David R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Utah Salt Lake City Utah USA"
}
],
"family": "Spivak",
"given": "Adam M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School Boston Massachusetts USA"
}
],
"family": "Sarwar",
"given": "Ammar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Laboratory Medicine and Pathology University of Washington Seattle Washington USA"
}
],
"family": "Johnston",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intermountain Health Care University of Utah Salt Lake City Utah USA"
}
],
"family": "Webb",
"given": "Brandon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Calgary Calgary Alberta Canada"
}
],
"family": "Hill",
"given": "Michael D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases & Global Public Health UC San Diego School of Medicine San Diego California USA"
}
],
"family": "Smith",
"given": "Davey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Hospital of Tübingen Tübingen Germany"
},
{
"name": "Centre de Recherches Médicales de Lambaréné Lambaréné Gabon"
}
],
"family": "Kremsner",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bill & Melinda Gates Medical Research Institute Cambridge Massachusetts USA"
}
],
"family": "Curran",
"given": "Marla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "MMS Holdings Canton Michigan USA"
}
],
"family": "Carter",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bill & Melinda Gates Medical Research Institute Cambridge Massachusetts USA"
}
],
"family": "Alexander",
"given": "Jim",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8675-2868",
"affiliation": [
{
"name": "Fight AIDS and Infectious Diseases Foundation Barcelona Spain"
}
],
"authenticated-orcid": false,
"family": "Corbacho",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, Department of Medicine University of Minnesota Minneapolis Minnesota USA"
}
],
"family": "Lee",
"given": "Todd C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases and International Medicine, Department of Medicine University of Minnesota Minneapolis Minnesota USA"
}
],
"family": "Hullsiek",
"given": "Katherine Huppler",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of General Internal Medicine McGill University Health Center Montreal Quebec Canada"
}
],
"family": "McDonald",
"given": "Emily G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2545-8504",
"affiliation": [
{
"name": "University of Utah Salt Lake City Utah USA"
}
],
"authenticated-orcid": false,
"family": "Hess",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School Boston Massachusetts USA"
}
],
"family": "Hughes",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Laboratory Medicine and Pathology University of Washington Seattle Washington USA"
}
],
"family": "Baeten",
"given": "Jared M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Alberta Edmonton Canada"
}
],
"family": "Schwartz",
"given": "Ilan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Calgary Calgary Alberta Canada"
}
],
"family": "Metz",
"given": "Luanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Alberta Edmonton Canada"
}
],
"family": "Richer",
"given": "Lawrence",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine David Geffen School of Medicine at University of California Los Angeles California USA"
}
],
"family": "Chew",
"given": "Kara W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lundquist Institute at Harbor‐UCLA Medical Center Torrance California USA"
}
],
"family": "Daar",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Medicine University of North Carolina at Chapel Hill Chapel Hill North Carolina USA"
}
],
"family": "Wohl",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bill & Melinda Gates Medical Research Institute Cambridge Massachusetts USA"
}
],
"family": "Dunne",
"given": "Michael",
"sequence": "additional"
}
],
"container-title": "Clinical and Translational Science",
"container-title-short": "Clinical Translational Sci",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
1,
5
]
],
"date-time": "2023-01-05T07:42:20Z",
"timestamp": 1672904540000
},
"deposited": {
"date-parts": [
[
2023,
1,
5
]
],
"date-time": "2023-01-05T07:42:31Z",
"timestamp": 1672904551000
},
"indexed": {
"date-parts": [
[
2023,
1,
5
]
],
"date-time": "2023-01-05T08:15:39Z",
"timestamp": 1672906539515
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
4
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
4
]
],
"date-time": "2023-01-04T00:00:00Z",
"timestamp": 1672790400000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
4
]
],
"date-time": "2023-01-04T00:00:00Z",
"timestamp": 1672790400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.13468",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/cts.13468",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.13468",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2023,
1,
4
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.5582/ddt.2020.01012",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1111/j.1432-1033.1983.tb07841.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1186/1743-422X-2-69",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1016/S1473-3099(06)70361-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1016/j.ijantimicag.2007.05.015",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.17925/EE.2020.16.2.109",
"article-title": "Short‐term hydroxychloroquine in COVID‐19 infection in people with or without metabolic syndrome–clearing safety issues and good clinical practice",
"author": "Dutta D",
"doi-asserted-by": "crossref",
"first-page": "109",
"journal-title": "Eur Endocrinol",
"key": "e_1_2_10_9_1",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.3390/ph15050634",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1161/CIRCEP.120.008688",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1056/NEJMoa2012410",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"article-title": "Mortality outcomes with hydroxychloroquine and chloroquine in COVID‐19 from an international collaborative meta‐analysis of randomized trials",
"author": "Axfors C",
"first-page": "1",
"journal-title": "Nat Commun",
"key": "e_1_2_10_13_1",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.22240",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.9778/cmajo.20210069",
"article-title": "Assessing the efficacy and safety of hydroxychloroquine as outpatient treatment of COVID‐19: a randomized controlled trial",
"author": "Schwartz I",
"doi-asserted-by": "crossref",
"first-page": "E693",
"journal-title": "C Open",
"key": "e_1_2_10_16_1",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100773",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1001/jamanetworkopen.2021.6468",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1093/cid/ciaa1009",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1056/NEJMoa2021801",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1056/NEJMoa2022926",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"article-title": "WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID‐19",
"author": "World Health Organization (WHO)",
"journal-title": "Dent News",
"key": "e_1_2_10_23_1",
"year": "2020"
},
{
"DOI": "10.36416/1806-3756/e20210236",
"article-title": "Use of hydroxychloroquine to prevent SARS‐CoV‐2 infection and treat mild COVID‐19: a systematic review and meta‐analysis",
"author": "Tanni S",
"doi-asserted-by": "crossref",
"first-page": "e20210236",
"journal-title": "J Bras Pneumol",
"key": "e_1_2_10_24_1",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1016/j.jiac.2021.02.021",
"article-title": "Efficacy and safety of hydroxychloroquine/chloroquine against SARS‐CoV‐2 infection: a systematic review and meta‐analysis",
"author": "Kumar J",
"doi-asserted-by": "crossref",
"first-page": "882",
"journal-title": "J Infect Chemother",
"key": "e_1_2_10_25_1",
"volume": "27",
"year": "2021"
},
{
"article-title": "Chloroquine or hydroxychloroquine for prevention and treatment of COVID‐19",
"author": "Singh B",
"first-page": "CD01358",
"journal-title": "Cochrane Database Syst Rev",
"key": "e_1_2_10_26_1",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkaa403",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1016/j.lana.2021.100062",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1001/jama.2015.3656",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/cts.13468"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics",
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine",
"General Neuroscience"
],
"subtitle": [],
"title": "Hydroxychloroquine for treatment of non‐hospitalized adults with\n <scp>COVID</scp>\n ‐19: A meta‐analysis of individual participant data of randomized trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
