A Retrospective Analysis of Azvudine in Patients with COVID-19 and Pre-existing Cancer
et al., Journal of Cancer, doi:10.7150/jca.91530, Mar 2024
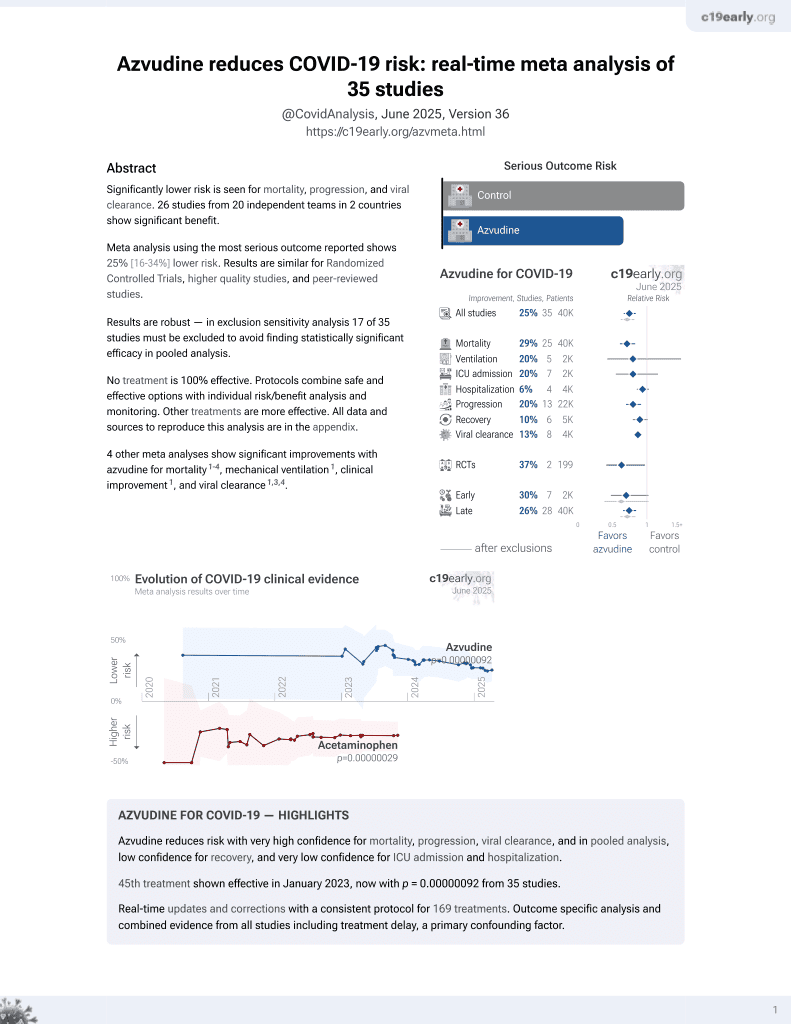
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
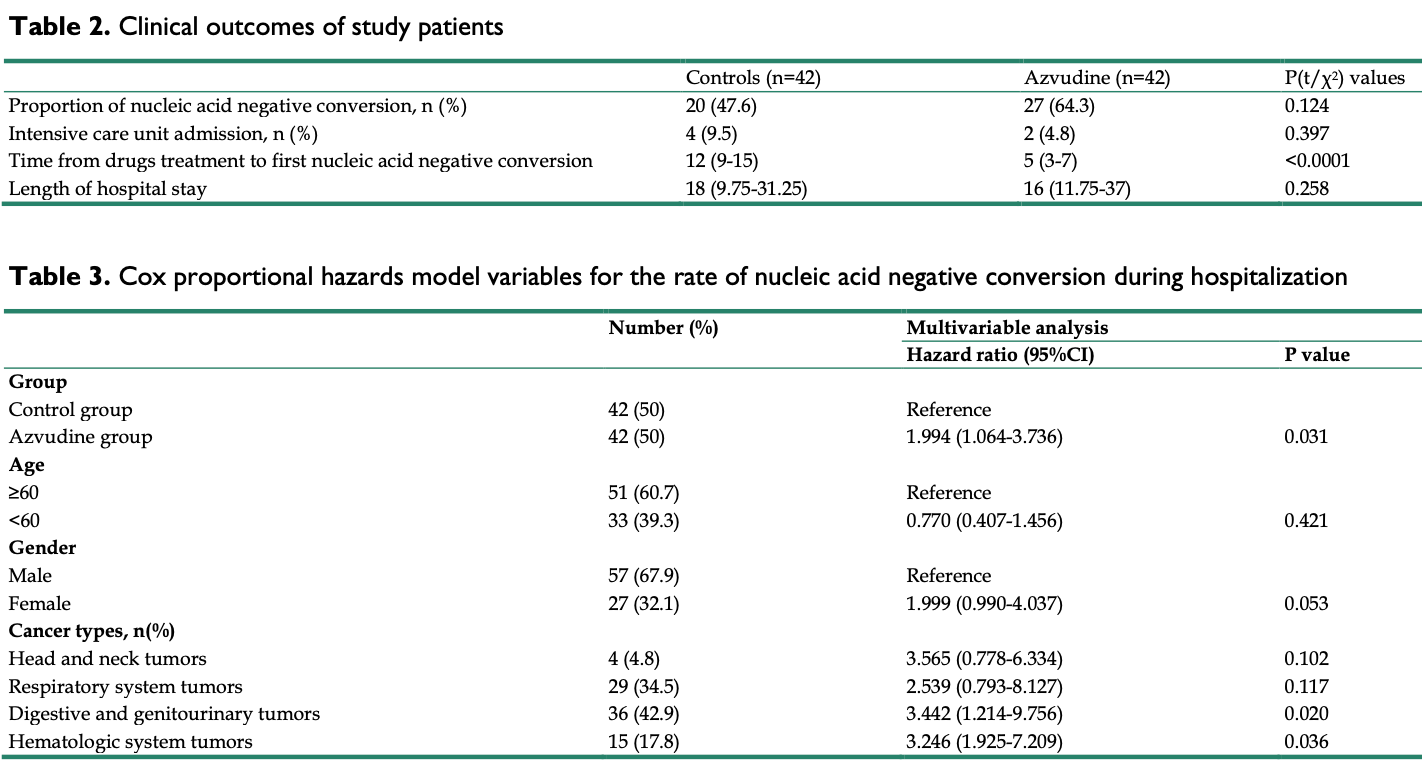
PSM retrospective 84 hospitalized COVID-19 patients with pre-existing cancer in China, showing faster viral clearance with azvudine. There was no significant difference in length of hospital stay or ICU admission.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of ICU admission, 50.0% lower, RR 0.50, p = 0.68, treatment 2 of 42 (4.8%), control 4 of 42 (9.5%), NNT 21.
|
|
hospitalization time, 11.1% lower, relative time 0.89, p = 0.26, treatment 42, control 42.
|
|
risk of no viral clearance, 49.8% lower, HR 0.50, p = 0.03, treatment 42, control 42, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
|
risk of no viral clearance, 31.8% lower, RR 0.68, p = 0.19, treatment 15 of 42 (35.7%), control 22 of 42 (52.4%), NNT 6.0.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Li et al., 4 Mar 2024, retrospective, China, peer-reviewed, 6 authors.
A Retrospective Analysis of Azvudine in Patients with COVID-19 and Pre-existing Cancer
Journal of Cancer, doi:10.7150/jca.91530
Objectives: Azvudine has been recommended as a potential treatment for the recently discovered Coronavirus disease in 2019. However, the effectiveness of Azvudine in individuals who have both COVID-19 and pre-existing cancer remains uncertain. Consequently, we undertook a retrospective analysis to evaluate the clinical efficacy of Azvudine therapy in hospitalized patients with COVID-19 and pre-existing cancer. Methods: This is a single-center retrospective analysis of patients diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, selected from patients admitted to a specialist oncology hospital between June 1, 2022 to June 31, 2023 with positive RT-PCR and pre-existing cancer. After exclusion and propensity score matching, patients in the test group treated with Azvudine and control patients treated with standard antiviral therapy were included. The primary outcome is the interval time from the first dose of Azvudine to the attainment of the first negative result for nucleic acid. Secondary outcomes included the rate of nucleic acid conversion, the duration of hospitalization, and the admission to the intensive care unit (ICU). Cox proportional hazards models were used to analyze the hazard ratio (HR) of event outcomes and to assess whether cancer types and Azvudine treatment will affect the course of COVID-19, specifically the time it takes for primary symptoms to alleviate. Results: In this study, a total of 84 patients were included for analysis. Among them, 42 patients received Azvudine treatment after hospitalization, and the rest were treated with standard antiviral therapy. The results expressed that the time taken for the first negative nucleic acid test was significantly shorter in the Azvudine group compared to the control group [5 (IQR3-7) d vs 12 (IQR9-15) d], p<0.0001. This difference was statistically significant. Furthermore, a multivariate COX analysis indicated that Azvudine treatment could effectively reduce the time required for nucleic acid conversion in cancer patients (HR 1.994, p=0.031). And the type of cancer also had an impact on the course of COVID-19 in patients. (HR 3.442, p=0.020; HR 3.246, p=0.036). Conclusion: Azvudine was correlated with a reduced duration for achieving nucleic acid conversion in individuals diagnosed with cancer. And different types of cancer have a certain impact on the course of COVID-19 for patients.
Project Plan (SD2023027).
Author contributions Conception and design of the research: FL, ZH, JY, and KZ. Analysis and interpretation of data: FL, KZ, YQ, and XK. Writing of the manuscript: FL, KZ, and ZH. Critical revision of the manuscript for intellectual content: JY and ZH. All authors read and approved the final draft. All authors contributed to the article and approved the submitted version.
Competing Interests The authors have declared that no competing interest exists.
References
Aboueshia, Hussein, Attia, Swinford, Miller et al., Cancer and COVID-19: analysis of patient outcomes, Future oncology
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Dai, Liu, Liu, Zhou, Li et al., Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak, Cancer discovery
Liang, Guan, Chen, Li, Xu, Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China, The Lancet Oncology
Moujaess, Kourie, Ghosn, Cancer patients and research during COVID-19 pandemic: A systematic review of current evidence, Critical reviews in oncology/hematology
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clinical infectious diseases : an official publication of the Infectious Diseases Society of America
Ren, Luo, Yu, Song, Liang et al., A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study
Su, Hu, Ke, Shi, Zheng et al., Pb2326: Outcomes of Anti-Cancer Therapy in Patients with Progressive Refractory/Relapsed B-Cell Lymphoma during Severe Coronavirus Disease 2019 (Covid-19): A Real-World Study from China: Hemasphere, eCollection, doi:10.1097/01.HS9.0000976024.45927.20
Sun, Dian, Shen, Zeng, Chen, Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, eClinicalMedicine
Wang, The First Domestic Original Drug for the Treatment of COVID-19: Azvudine, Chinese Pharmaceutical Journal
Wang, Yang, Luo, Peng, Dai et al., Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro, PloS one
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduction and Targeted Therapy
DOI record:
{
"DOI": "10.7150/jca.91530",
"ISSN": [
"1837-9664"
],
"URL": "http://dx.doi.org/10.7150/jca.91530",
"author": [
{
"affiliation": [],
"family": "Li",
"given": "Fangyu",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Keao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qi",
"given": "Xueyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cui",
"given": "Kaixia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hao",
"given": "Zhiying",
"sequence": "additional"
}
],
"container-title": "Journal of Cancer",
"container-title-short": "J. Cancer",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
3,
14
]
],
"date-time": "2024-03-14T02:37:24Z",
"timestamp": 1710383844000
},
"deposited": {
"date-parts": [
[
2024,
3,
14
]
],
"date-time": "2024-03-14T02:37:58Z",
"timestamp": 1710383878000
},
"indexed": {
"date-parts": [
[
2024,
3,
15
]
],
"date-time": "2024-03-15T00:33:31Z",
"timestamp": 1710462811841
},
"is-referenced-by-count": 0,
"issue": "8",
"issued": {
"date-parts": [
[
2024
]
]
},
"journal-issue": {
"issue": "8"
},
"language": "en",
"link": [
{
"URL": "https://www.jcancer.org/v15p2442.htm",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4137",
"original-title": [],
"page": "2442-2447",
"prefix": "10.7150",
"published": {
"date-parts": [
[
2024
]
]
},
"published-print": {
"date-parts": [
[
2024
]
]
},
"publisher": "Ivyspring International Publisher",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.jcancer.org/v15p2442.htm"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Oncology"
],
"subtitle": [],
"title": "A Retrospective Analysis of Azvudine in Patients with COVID-19 and Pre-existing Cancer",
"type": "journal-article",
"volume": "15"
}