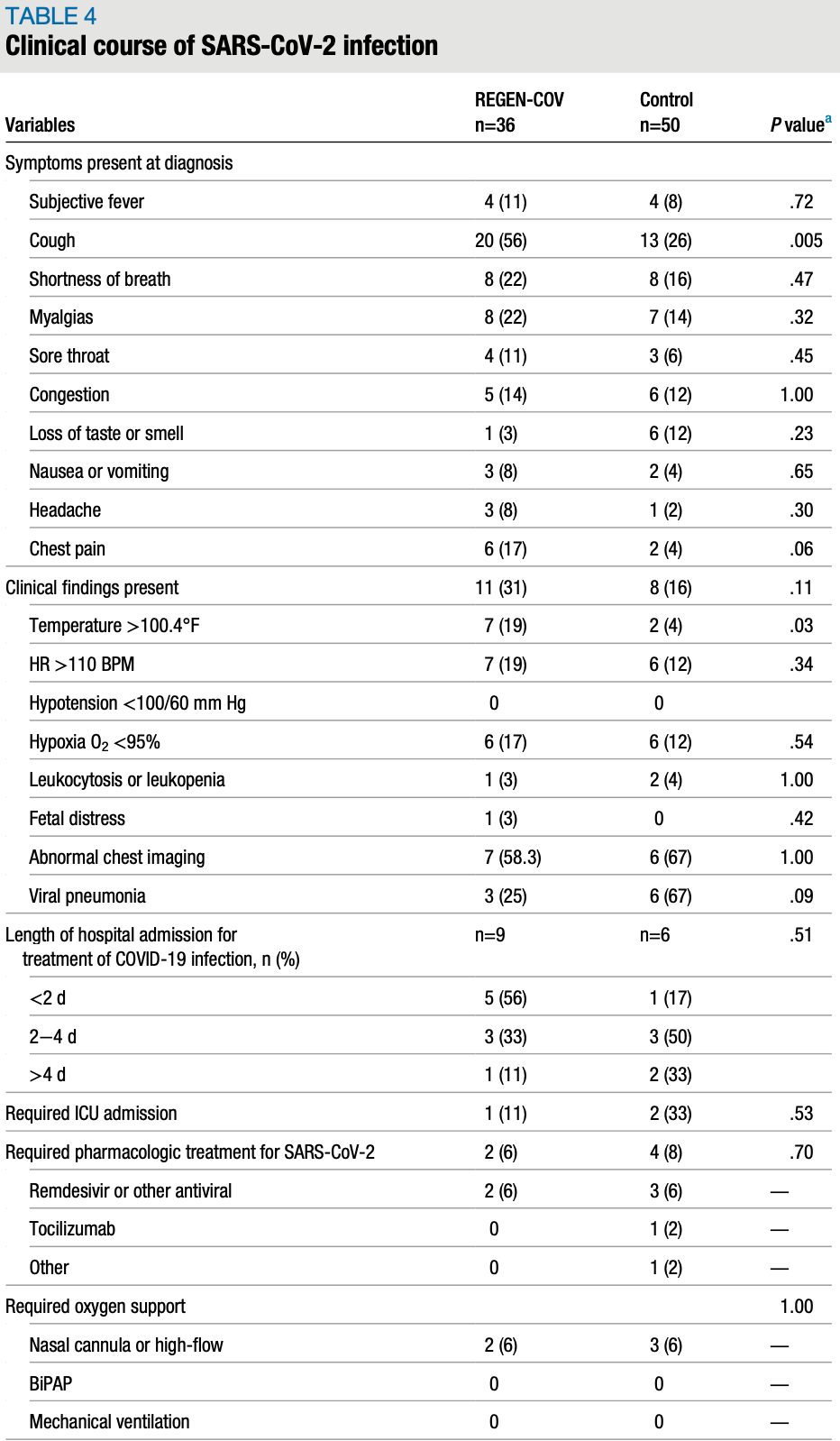
Outcomes of pregnant patients treated with REGEN-COV during the COVID-19 pandemic
et al., American Journal of Obstetrics & Gynecology MFM, doi:10.1016/j.ajogmf.2022.100673, Jun 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 86 pregnant COVID-19 patients, 36 treated with casirivimab/imdevimab, showing no significant difference in COVID-19 outcomes with treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of ICU admission, 30.6% lower, RR 0.69, p = 1.00, treatment 1 of 36 (2.8%), control 2 of 50 (4.0%), NNT 82.
|
|
risk of oxygen therapy, 7.4% lower, RR 0.93, p = 1.00, treatment 2 of 36 (5.6%), control 3 of 50 (6.0%), NNT 225.
|
|
risk of hospitalization, 108.3% higher, RR 2.08, p = 0.15, treatment 9 of 36 (25.0%), control 6 of 50 (12.0%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Levey et al., 4 Jun 2022, retrospective, USA, peer-reviewed, 6 authors, study period March 2021 - October 2021.
Outcomes of pregnant patients treated with REGEN-COV during the COVID-19 pandemic
American Journal of Obstetrics & Gynecology MFM, doi:10.1016/j.ajogmf.2022.100673
BACKGROUND: Pregnant patients with SARS-CoV-2 infection are at increased risk for severe disease including hospitalization, intensive care admission, ventilatory support, and death. Although pregnant patients were excluded from investigational trials for pharmacologic treatments for COVID-19 illness, the National Institutes of Health treatment guidelines state that efficacious treatments should not be withheld from pregnant patients. An infusion of casirivimab and imdevimab (REGEN-COV), a monoclonal antibody therapy, was shown to reduce the risk of COVID-19− related hospitalization or death from any cause and resolved symptoms and reduced SARS-CoV-2 viral load more rapidly than placebo. In July of 2021, the Food and Drug Administration released an Emergency Use Authorization for REGEN-COV. Although pregnant persons were not included in the original trials, given the higher risk of morbidity and mortality in the pregnant population, our institution offered REGEN-COV to our pregnant patients beginning in August of 2021. Side effects after REGEN-COV administration are rare and thought to be secondary to COVID-19 rather than REGEN-COV. OBJECTIVE: This study aimed to track safety and clinical outcomes in unvaccinated pregnant patients who received REGEN-COV and to compare these outcomes with those of a contemporary cohort of patients who tested positive for SARS-CoV-2 and were eligible but did not receive REGEN-COV. Our hypothesis was that REGEN-COV administration during pregnancy is safe, and that pregnant persons who received REGEN-COV would experience less severe COVID-19 respiratory illness, with decreased length of hospital stay, rates of intensive care unit admission, and need for oxygen and other COVID-19 therapeutics. STUDY DESIGN: This is a retrospective cohort study of pregnant patients who either tested positive for SARS-CoV-2 or had a known exposure to a COVID-19−positive person, and were therefore eligible for REGEN-COV at our institution. Within this cohort, we compared those who received REGEN-COV with those who did not between March and October of 2021 at Grady Memorial Hospital in Atlanta, Georgia. The main outcomes studied were perinatal outcomes, safety data, and the clinical course of SARS-CoV-2 infection. RESULTS: From March to October of 2021, 86 pregnant people tested positive for SARS-CoV-2 via real-time polymerase chain reaction or had a confirmed exposure. In this group, 36 received REGEN-COV and 50 did not. There were no instances of infusion rate adjustment or discontinuation, anaphylaxis, or death among individuals who received REGEN-COV. One individual experienced worsening shortness of breath >24 hours after administration, which was classified as an infusion-related reaction. There were no significant differences in perinatal outcomes, length of hospitalization, rates of intensive care unit admission, additional pharmacologic treatment for COVID-19, or oxygen requirement between the 2 groups. CONCLUSION:..
Author and article information From the Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, GA (Drs Levey, Forrest, Spielman, Dude, and Badell); Department of Biostatistics and Bioinformatics, Rollins School of Public Health, Emory University, Atlanta, GA (Mr Easley). Received Mar. 30, 2022; revised May 13, 2022; accepted June 1, 2022. The authors report no conflict of interest.
References
Copin, Baum, Wloga, The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies, Cell
Deeks, Casirivimab/imdevimab: first approval, Drugs
Desisto, Wallace, Simeone, Risk for stillbirth among women with and without COVID-19 at delivery hospitalization -United States, March 2020-September 2021, MMWR Morb Mortal Wkly Rep
Gurol-Urganci, Jardine, Carroll, Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study, Am J Obstet Gynecol
Halscott, Vaught, Society for Maternal-Fetal Medicine Management Considerations for Pregnant Patients With COVID-19
Hirshberg, Cooke, Oakes, Odibo, Raghuraman et al., Monoclonal antibody treatment of symptomatic COVID-19 in pregnancy: initial report, Am J Obstet Gynecol
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Mayer, Vanhise, Caskey, Naqvi, Burwick, Monoclonal antibodies casirivimab and imdevimab in pregnancy for coronavirus disease 2019 (COVID-19), Obstet Gynecol
Merison, Goldman, Bomze, Subcutaneous REGEN-COV antibody combination to prevent Covid-19, N Engl J Med
Metz, Clifton, Hughes, Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19), Obstet Gynecol
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention, medRxiv, doi:10.1101/2021.06.14.21258567
Richley, Rao, Afshar, Neutralizing monoclonal antibodies for coronavirus disease 2019 (COVID-19) in pregnancy: a case series, Obstet Gynecol
Stokes, Zambrano, Anderson, Coronavirus disease 2019 case surveillance -United States, January 22, MMWR Morb Mortal Wkly Rep
Thompson, Nguyen, Noble, Aronoff, COVID-19-related disease severity in pregnancy, Am J Reprod Immunol
Wang, Berry, Moutos, Association of the Delta (B.1.617.2) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with pregnancy outcomes, Obstet Gynecol
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Zambrano, Ellington, Strid, Update: characteristics of symptomatic women of reproductive age with laboratoryconfirmed SARS-CoV-2 infection by pregnancy status -United States, January 22, MMWR Morb Mortal Wkly Rep
DOI record:
{
"DOI": "10.1016/j.ajogmf.2022.100673",
"ISSN": [
"2589-9333"
],
"URL": "http://dx.doi.org/10.1016/j.ajogmf.2022.100673",
"alternative-id": [
"S2589933322001082"
],
"article-number": "100673",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Outcomes of pregnant patients treated with REGEN-COV during the COVID-19 pandemic"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "American Journal of Obstetrics & Gynecology MFM"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ajogmf.2022.100673"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Inc. All rights reserved."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7899-877X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Levey",
"given": "Natalie H.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Forrest",
"given": "Alexandra D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9433-2134",
"affiliation": [],
"authenticated-orcid": false,
"family": "Spielman",
"given": "Daniella W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Easley",
"given": "Kirk A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dude",
"given": "Carolynn M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badell",
"given": "Martina L.",
"sequence": "additional"
}
],
"container-title": "American Journal of Obstetrics & Gynecology MFM",
"container-title-short": "American Journal of Obstetrics & Gynecology MFM",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ajogmfm.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
6,
4
]
],
"date-time": "2022-06-04T06:06:27Z",
"timestamp": 1654322787000
},
"deposited": {
"date-parts": [
[
2022,
7,
24
]
],
"date-time": "2022-07-24T08:11:57Z",
"timestamp": 1658650317000
},
"funder": [
{
"DOI": "10.13039/100012182",
"doi-asserted-by": "publisher",
"name": "Emory Medical Care Foundation"
}
],
"indexed": {
"date-parts": [
[
2022,
7,
24
]
],
"date-time": "2022-07-24T08:40:44Z",
"timestamp": 1658652044150
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
9
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2022,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
1
]
],
"date-time": "2022-09-01T00:00:00Z",
"timestamp": 1661990400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589933322001082?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589933322001082?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100673",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
9
]
]
},
"published-print": {
"date-parts": [
[
2022,
9
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.ajogmf.2022.100673_bib0001",
"unstructured": "National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2021. Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed November 29, 2021."
},
{
"DOI": "10.1111/aji.13339",
"article-title": "COVID-19-related disease severity in pregnancy",
"author": "Thompson",
"doi-asserted-by": "crossref",
"first-page": "e13339",
"journal-title": "Am J Reprod Immunol",
"key": "10.1016/j.ajogmf.2022.100673_bib0002",
"volume": "84",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.1016/j.ajogmf.2022.100673_bib0003",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6924e2",
"article-title": "Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020",
"author": "Stokes",
"doi-asserted-by": "crossref",
"first-page": "759",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.ajogmf.2022.100673_bib0004",
"volume": "69",
"year": "2020"
},
{
"key": "10.1016/j.ajogmf.2022.100673_bib0005",
"unstructured": "Centers for Disease Control and Prevention. Pregnant and recently pregnant people at increased risk for severe illness from COVID-19. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html#print. Accessed November 29, 2021."
},
{
"DOI": "10.15585/mmwr.mm6944e3",
"article-title": "Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020",
"author": "Zambrano",
"doi-asserted-by": "crossref",
"first-page": "1641",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.ajogmf.2022.100673_bib0006",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1097/AOG.0000000000004689",
"article-title": "Neutralizing monoclonal antibodies for coronavirus disease 2019 (COVID-19) in pregnancy: a case series",
"author": "Richley",
"doi-asserted-by": "crossref",
"first-page": "368",
"journal-title": "Obstet Gynecol",
"key": "10.1016/j.ajogmf.2022.100673_bib0007",
"volume": "139",
"year": "2022"
},
{
"DOI": "10.1097/AOG.0000000000004339",
"article-title": "Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19)",
"author": "Metz",
"doi-asserted-by": "crossref",
"first-page": "571",
"journal-title": "Obstet Gynecol",
"key": "10.1016/j.ajogmf.2022.100673_bib0008",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7047e1",
"article-title": "Risk for stillbirth among women with and without COVID-19 at delivery hospitalization - United States, March 2020-September 2021",
"author": "DeSisto",
"doi-asserted-by": "crossref",
"first-page": "1640",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.ajogmf.2022.100673_bib0009",
"volume": "70",
"year": "2021"
},
{
"key": "10.1016/j.ajogmf.2022.100673_bib0010",
"unstructured": "Halscott T, Vaught J. Society for Maternal-Fetal Medicine Management Considerations for Pregnant Patients With COVID-19. SMFM Covid Clinical. Published February 2, 2021. https://www.smfm.org/covidclinical. Accessed November 29, 2021."
},
{
"key": "10.1016/j.ajogmf.2022.100673_bib0011",
"unstructured": "The American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetricians-gynecologists, obstetrics. 2020. Available at: https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics. Accessed November 29, 2021."
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajogmf.2022.100673_bib0012",
"volume": "384",
"year": "2021"
},
{
"key": "10.1016/j.ajogmf.2022.100673_bib0013",
"unstructured": "COVID-19 study assessing the efficacy and safety of anti-spike SARS CoV-2 monoclonal antibodies for prevention of SARS CoV-2 infection asymptomatic in healthy adults and adolescents who are household contacts to an individual with a positive SARS-CoV-2 RT-PCR assay. Clin Trials.gov."
},
{
"DOI": "10.1016/j.cell.2021.06.002",
"article-title": "The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies",
"author": "Copin",
"doi-asserted-by": "crossref",
"first-page": "3949",
"journal-title": "Cell",
"key": "10.1016/j.ajogmf.2022.100673_bib0014",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajogmf.2022.100673_bib0015",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1007/s40265-021-01620-z",
"article-title": "Casirivimab/imdevimab: first approval",
"author": "Deeks",
"doi-asserted-by": "crossref",
"first-page": "2047",
"journal-title": "Drugs",
"key": "10.1016/j.ajogmf.2022.100673_bib0016",
"volume": "81",
"year": "2021"
},
{
"key": "10.1016/j.ajogmf.2022.100673_bib0017",
"unstructured": "US Food and Drug Administration. FDA authorizes REGEN-COV monoclonal antibody therapy for post-exposure prophylaxis for COVID-19. 2021. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-bamlanivimab-and-etesevimab-monoclonal-antibody-therapy-post-exposure-prophylaxis. Accessed November 29, 2021."
},
{
"key": "10.1016/j.ajogmf.2022.100673_bib0018",
"unstructured": "US Food and Drug Administration. Fact sheet for health care providersemergency use authorization (EUA) of REGEN-COV. 2022. Available at: https://www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf. Accessed November 29, 2021."
},
{
"DOI": "10.1097/AOG.0000000000004603",
"article-title": "Monoclonal antibodies casirivimab and imdevimab in pregnancy for coronavirus disease 2019 (COVID-19)",
"author": "Mayer",
"doi-asserted-by": "crossref",
"first-page": "937",
"journal-title": "Obstet Gynecol",
"key": "10.1016/j.ajogmf.2022.100673_bib0019",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.1016/j.ajog.2021.08.025",
"article-title": "Monoclonal antibody treatment of symptomatic COVID-19 in pregnancy: initial report",
"author": "Hirshberg",
"doi-asserted-by": "crossref",
"first-page": "688",
"journal-title": "Am J Obstet Gynecol",
"key": "10.1016/j.ajogmf.2022.100673_bib0020",
"volume": "225",
"year": "2021"
},
{
"DOI": "10.1101/2021.06.14.21258567",
"doi-asserted-by": "crossref",
"key": "10.1016/j.ajogmf.2022.100673_bib0021",
"unstructured": "O'Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV Antibody Combination for Covid-19 Prevention. medRxiv. Jun 17 2021;doi:10.1101/2021.06.14.21258567"
},
{
"DOI": "10.1056/NEJMc2113862",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent Covid-19",
"author": "Merison",
"doi-asserted-by": "crossref",
"first-page": "e70",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ajogmf.2022.100673_bib0022",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1097/AOG.0000000000004595",
"article-title": "Association of the Delta (B.1.617.2) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with pregnancy outcomes",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "838",
"journal-title": "Obstet Gynecol",
"key": "10.1016/j.ajogmf.2022.100673_bib0023",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.1016/j.ajog.2021.05.016",
"article-title": "Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study",
"author": "Gurol-Urganci",
"doi-asserted-by": "crossref",
"first-page": "522",
"journal-title": "Am J Obstet Gynecol",
"key": "10.1016/j.ajogmf.2022.100673_bib0024",
"volume": "225",
"year": "2021"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589933322001082"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine",
"Obstetrics and Gynecology"
],
"subtitle": [],
"title": "Outcomes of pregnant patients treated with REGEN-COV during the COVID-19 pandemic",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "4"
}
