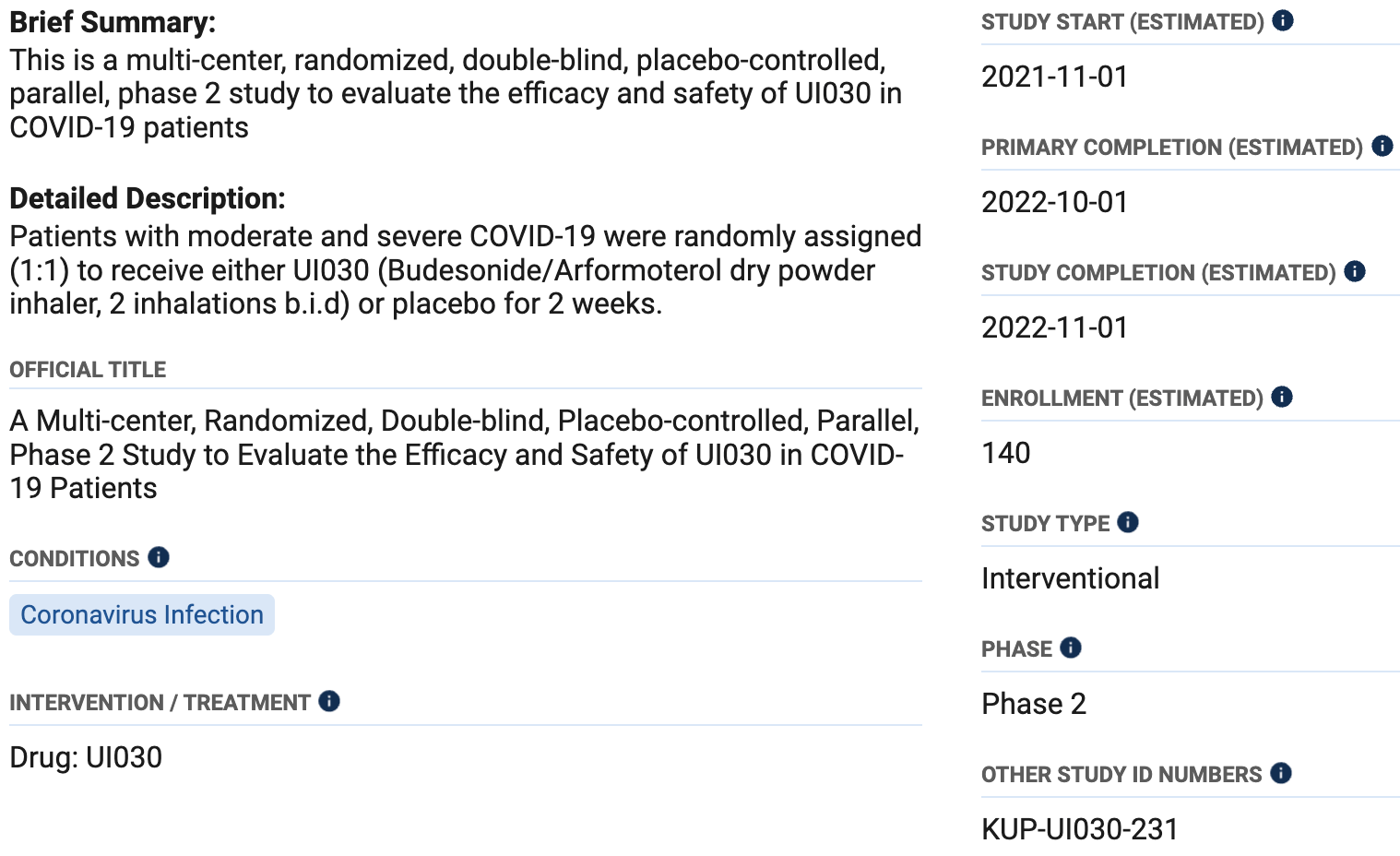
A Multi-center, Randomized, Double-blind, Placebo-controlled, Parallel, Phase 2 Study to Evaluate the Efficacy and Safety of UI030 in COVID-19 Patients
, NCT05055414, NCT05055414, Nov 2022
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Estimated 140 patient budesonide early treatment RCT with results not reported over 3 years after estimated completion.
1.
Marcy et al., Early Treatment of Vulnerable Individuals With Non-Severe SARS-CoV-2 Infection: A Multi-Arm Multi-Stage Randomized Trial (MAMS) to Evaluate the Effectiveness of Several Specific Treatments in Reducing the Risk of Clinical Worsening or Death in Sub-Saharan Africa (COVERAGE-Africa), NCT04920838, clinicaltrials.gov/study/NCT04920838.
2.
Korea United Pharm., A Multi-center, Randomized, Double-blind, Placebo-controlled, Parallel, Phase 2 Study to Evaluate the Efficacy and Safety of UI030 in COVID-19 Patients, NCT05055414, clinicaltrials.gov/study/NCT05055414.
Korea United Pharm. et al., 1 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, South Korea, trial NCT05055414 (history).
