The effect of casirivimab with imdevimab on disease progression in nonsevere COVID-19 patients in a single hospital in Japan
et al., Journal of General and Family Medicine, doi:10.1002/jgf2.516, Dec 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Combined retrospective/prospective study in Japan with 53 casirivimab/imdevimab patients and 75 control patients, showing significantly lower progression with treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
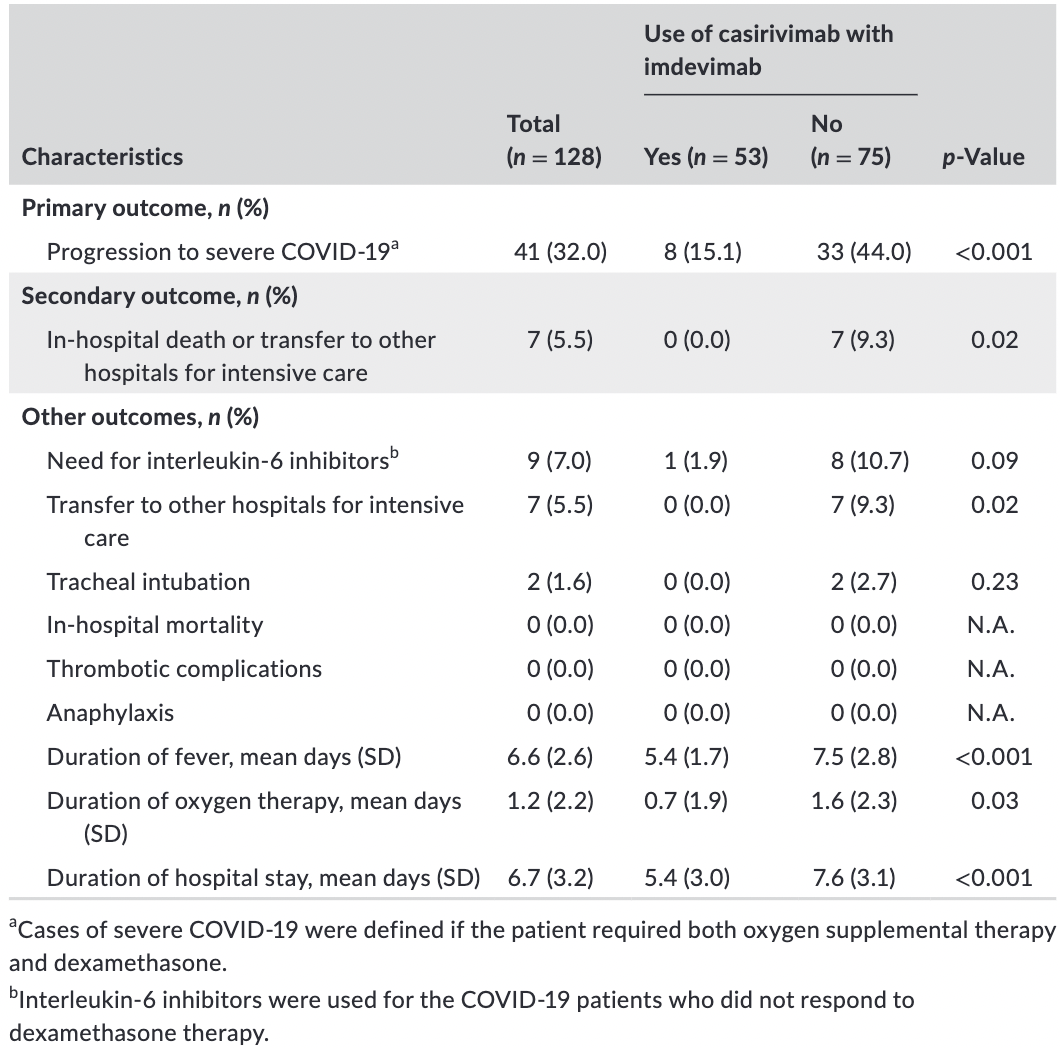
risk of mechanical ventilation, 77.3% lower, RR 0.23, p = 0.51, treatment 0 of 53 (0.0%), control 2 of 75 (2.7%), NNT 38, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 92.3% lower, RR 0.08, p = 0.04, treatment 0 of 53 (0.0%), control 7 of 75 (9.3%), NNT 11, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of progression, 67.8% lower, RR 0.32, p = 0.006, treatment 8 of 53 (15.1%), control 33 of 75 (44.0%), NNT 3.5, adjusted per study, odds ratio converted to relative risk, multivariable, primary outcome.
|
|
hospitalization time, 28.9% lower, relative time 0.71, p < 0.001, treatment 53, control 75.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Komagamine et al., 19 Dec 2021, retrospective, Japan, peer-reviewed, 4 authors, average treatment delay 5.0 days.
The effect of casirivimab with imdevimab on disease progression in nonsevere COVID‐19 patients in a single hospital in Japan
Journal of General and Family Medicine, doi:10.1002/jgf2.516
Background: Recent randomized trials have revealed that neutralizing monoclonal antibodies can reduce disease progression in mild-moderate COVID-19 patients. However, no studies have investigated the effect of neutralizing monoclonal antibodies on clinical outcomes in Japan. Methods: A single-center retrospective and prospective cohort study was conducted. All consecutive febrile nonsevere COVID-19 patients with at least one risk factor were included. The primary outcome was progression to severe COVID-19. Severe COVID-19 cases were defined as patients requiring oxygen therapy and dexamethasone. The differences in the primary outcomes between the patients who were treated with casirivimab with imdevimab (treatment group) and those who were not (control group) were compared using the chi-squared test. Results: A total of 128 patients were included. Of those, the mean age was 53.6 years old (SD 9.9), and 52 (40.6%) were women. Fifty-three patients were treated with casirivimab with imdevimab, and 75 patients were given the standard treatment only. The primary outcome occurred in eight (15.1%) of the 53 patients in the treatment group and 33 (44.0%) of the 75 patients in the control group (odd ratio [OR] 0.23, 95% CI 0.09 to 0.55). The multivariate analysis revealed that the use of casirivimab with imdevimab (OR 0.21, 95% CI 0.08 to 0.54) was the only independent risk factor associated with progression to severe COVID-19. No patients died during hospitalization in either group.
Conclusion: Similar to other countries, casirivimab with imdevimab significantly reduced disease progression in early nonsevere COVID-19 patients with fever and risk factors in Japan.
References
Charlson, Pompei, Ales, Mackenzie, A new method of classifying prognostic comorbidity in longitudinal studies: development and validation, J Chronic Dis
Cohen, Nirula, Mulligan, Novak, Marovich et al., Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized controlled trial, JAMA
Dagan, Barda, Keptan, Miron, Perchik et al., BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting, N Engl J Med
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab plus etesevimab in mild or moderate covid-19, N Engl J Med
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1101/2021.05.27.21257096
Matsunaga, Hayakawa, Terada, Ohtsu, Asai et al., Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 Registry Japan, Clin Infect Dis, doi:10.1093/cid/ciaa1470
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Subcutaneous REGEN-COV antibody combination to prevent covid-19, N Engl J Med
Peduzzi, Concato, Kemper, Holford, Feinstein, A simulation study of the number of events per variable in logistic regression analysis, J Clin Epidemiol
Pharmaceuticals, Devices, RONAPREVE for intravenous infusion set 300/ RONAPREVE for intravenous infusion set 1332
Roth, Emmons-Bell, Alger, Bradley, Das et al., Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic, JAMA Network Open
Siemieniuk, Bartoszko, Ge, Zeraatkar, Izcovich et al., Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat Rev Immunol
Tian, Jiang, Tao, Nicholson, Li et al., Predictor of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis, J Med Virol
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Zhu, Zhang, Li, Yang, Song, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.1002/jgf2.516",
"ISSN": [
"2189-7948",
"2189-7948"
],
"URL": "http://dx.doi.org/10.1002/jgf2.516",
"alternative-id": [
"10.1002/jgf2.516"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-10-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-12-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-12-19"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5899-4760",
"affiliation": [
{
"name": "Department of Internal Medicine National Hospital Organization Tochigi Medical Center Utsunomiya Japan"
}
],
"authenticated-orcid": false,
"family": "Komagamine",
"given": "Junpei",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine National Hospital Organization Tochigi Medical Center Utsunomiya Japan"
}
],
"family": "Yabuki",
"given": "Taku",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine National Hospital Organization Tochigi Medical Center Utsunomiya Japan"
}
],
"family": "Yoshihara",
"given": "Satsuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine National Hospital Organization Tochigi Medical Center Utsunomiya Japan"
}
],
"family": "Tanaka",
"given": "Nao",
"sequence": "additional"
}
],
"container-title": [
"Journal of General and Family Medicine"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
20
]
],
"date-time": "2021-12-20T05:33:13Z",
"timestamp": 1639978393000
},
"deposited": {
"date-parts": [
[
2021,
12,
20
]
],
"date-time": "2021-12-20T05:33:19Z",
"timestamp": 1639978399000
},
"indexed": {
"date-parts": [
[
2021,
12,
21
]
],
"date-time": "2021-12-21T06:44:37Z",
"timestamp": 1640069077884
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2189-7948"
},
{
"type": "electronic",
"value": "2189-7948"
}
],
"issued": {
"date-parts": [
[
2021,
12,
19
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
19
]
],
"date-time": "2021-12-19T00:00:00Z",
"timestamp": 1639872000000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
19
]
],
"date-time": "2021-12-19T00:00:00Z",
"timestamp": 1639872000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jgf2.516",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/jgf2.516",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jgf2.516",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
12,
19
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
19
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_2_1"
},
{
"DOI": "10.1136/bmj.m2980",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_3_1"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_4_1"
},
{
"DOI": "10.1001/jama.2021.8828",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_5_1"
},
{
"DOI": "10.1056/NEJMoa2109682",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_6_1"
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_7_1"
},
{
"DOI": "10.1056/NEJMoa2102685",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_8_1"
},
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_9_1"
},
{
"DOI": "10.1093/cid/ciaa1470",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_10_1"
},
{
"key": "e_1_2_6_11_1",
"unstructured": "Pharmaceuticals and Medical Devices Agency.RONAPREVE for intravenous infusion set 300/ RONAPREVE for intravenous infusion set 1332 (in Japanese).https://www.info.pmda.go.jp/go/pack/62505A0A1023_1_01/Accessed October 12 2021."
},
{
"DOI": "10.1016/0021-9681(87)90171-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_12_1"
},
{
"DOI": "10.1002/jmv.26050",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_13_1"
},
{
"DOI": "10.1001/jamanetworkopen.2021.8828",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_14_1"
},
{
"DOI": "10.1056/NEJMoa2101765",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_15_1"
},
{
"article-title": "Casirivimab and imdevimab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial",
"author": "RECOVERY Collaborative Group",
"journal-title": "medRxiv",
"key": "e_1_2_6_16_1",
"year": "2021"
},
{
"DOI": "10.1016/S0895-4356(96)00236-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_6_17_1"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"score": 1,
"short-container-title": [
"J of Gen and Family Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Family Practice",
"Geriatrics and Gerontology",
"Internal Medicine"
],
"subtitle": [],
"title": [
"The effect of casirivimab with imdevimab on disease progression in nonsevere COVID‐19 patients in a single hospital in Japan"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
