Serious Clinical Outcomes of COVID-19 Related to Acetaminophen or NSAIDs from a Nationwide Population-Based Cohort Study
et al., International Journal of Environmental Research and Public Health, doi:10.3390/ijerph20053832, Feb 2023
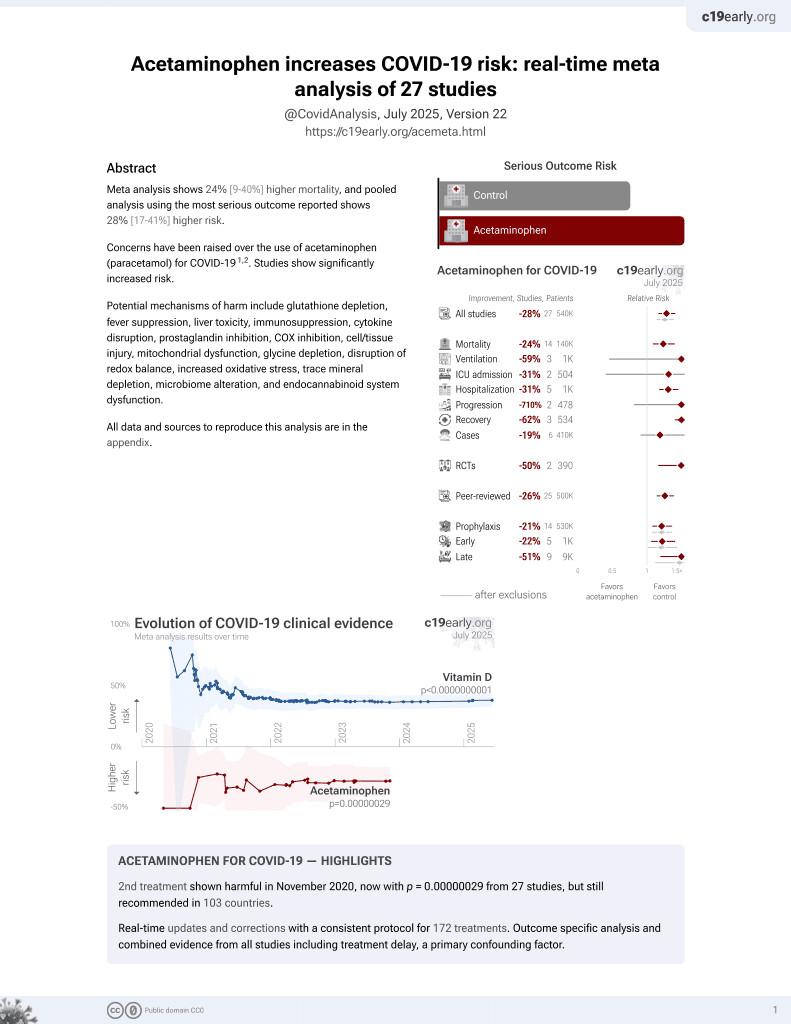
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
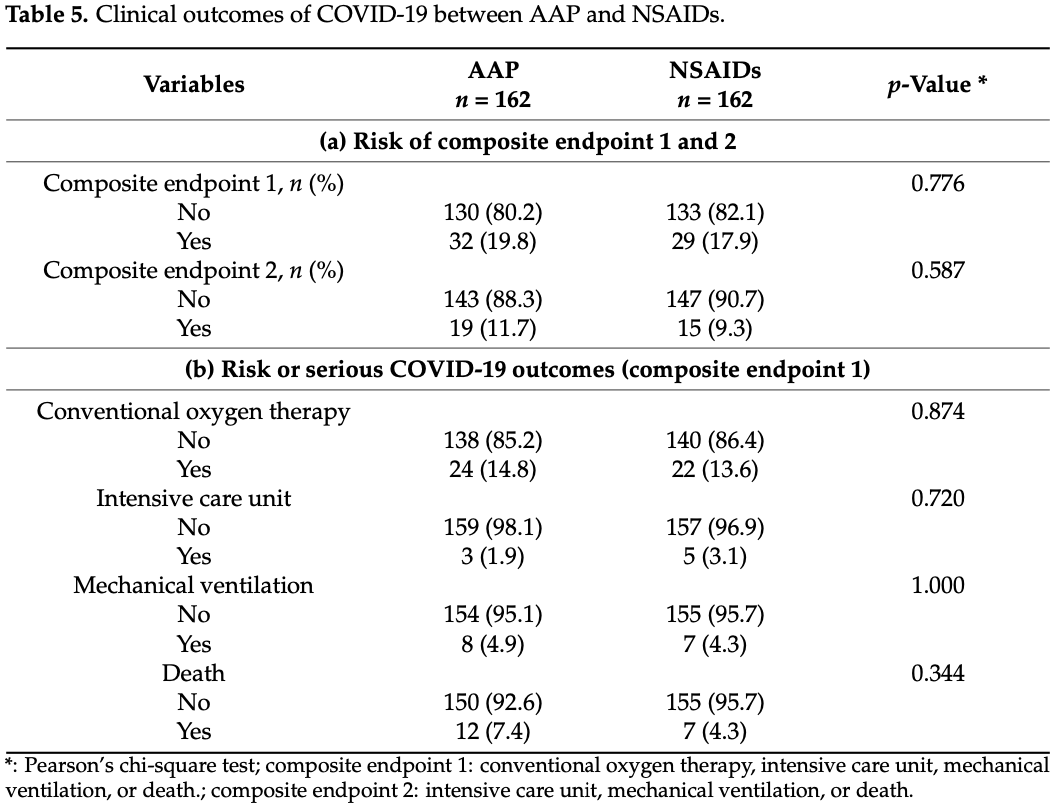
PSM retrospective in South Korea, showing no significant differences in outcomes with acetaminophen use vs. NSAID use. Adherence and dosage are unknown.
Acetaminophen is also known as paracetamol, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
|
risk of death, 71.4% higher, RR 1.71, p = 0.34, treatment 12 of 162 (7.4%), control 7 of 162 (4.3%), propensity score matching.
|
|
risk of mechanical ventilation, 14.3% higher, RR 1.14, p = 1.00, treatment 8 of 162 (4.9%), control 7 of 162 (4.3%), propensity score matching.
|
|
risk of ICU admission, 40.0% lower, RR 0.60, p = 0.72, treatment 3 of 162 (1.9%), control 5 of 162 (3.1%), NNT 81, propensity score matching.
|
|
risk of oxygen therapy, 9.1% higher, RR 1.09, p = 0.87, treatment 24 of 162 (14.8%), control 22 of 162 (13.6%), propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kim et al., 21 Feb 2023, retrospective, South Korea, peer-reviewed, mean age 55.8, 4 authors, this trial compares with another treatment - results may be better when compared to placebo.
Contact: lsceline78@gmail.com (corresponding author).
Serious Clinical Outcomes of COVID-19 Related to Acetaminophen or NSAIDs from a Nationwide Population-Based Cohort Study
International Journal of Environmental Research and Public Health, doi:10.3390/ijerph20053832
Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) have been widely prescribed to infected patients; however, the safety of them has not been investigated in patients with serious acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Our objective was to evaluate the association between the previous use of acetaminophen or NSAIDs and the clinical outcomes of SARS-CoV-2 infection. A nationwide population-based cohort study was conducted using the Korean Health Insurance Review and Assessment Database through propensity score matching (PSM). A total of 25,739 patients aged 20 years and older who tested for SARS-CoV-2 were included from 1 January 2015 to 15 May 2020. The primary endpoint was a positive result for a SARS-CoV-2 test, and the secondary endpoint was serious clinical outcomes of SARS-CoV-2 infection, such as conventional oxygen therapy, admission to the intensive care unit, need for invasive ventilation care, or death. Of 1058 patients, after propensity score matching, 176 acetaminophen users and 162 NSAIDs users were diagnosed with coronavirus disease 2019. After PSM, 162 paired data sets were generated, and the clinical outcomes of the acetaminophen group were not significantly different from those of the NSAIDs group. This suggests that acetaminophen and NSAIDs can be used safely to control symptoms in patients suspected of having SARS-CoV-2.
Author Contributions: Conceptualization, J.-W.K. and S.L.; methodology, S.Y.; software S.Y.; validation, S.L.; formal analysis, S.Y.; investigation, J.-W.K. and J.L.; resources, S.L.; data curation, S.Y.; writing-original draft preparation, J.-W.K. and S.Y.; writing-review and editing, S.L.; visualization, J.L.; supervision, S.L.; project administration, S.L. All authors have read and agreed to the published version of the manuscript. Informed Consent Statement: Informed consent was obtained from the patients to publish this paper.
Conflicts of Interest: The authors declare no conflict of interest.
References
Amponsah, Tagoe, Adams, Bugyei, Efficacy and safety profile of corticosteroids and non-steroidal antiinflammatory drugs in COVID-19 management: A narrative review, Front. Pharmacol, doi:10.3389/fphar.2022.1063246
Basille, Thomsen, Madsen, Duhaut, Andrejak et al., Nonsteroidal Antiinflammatory Drug Use and Clinical Outcomes of Community-acquired Pneumonia, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.201802-0229LE
Bhala, Emberson, Merhi, Abramson, Arber et al., Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials, Lancet, doi:10.1016/s0140-6736(13)60900-9
Blanco, Ambrosioni, Garcia, Martínez, Soriano et al., COVID-19 in patients with HIV: Clinical case series, Lancet HIV, doi:10.1016/S2352-3018(20)30111-9
Bruno, Tacconelli, Patrignani, Variability in the response to non-steroidal anti-inflammatory drugs: Mechanisms and perspectives, Basic Clin. Pharmacol. Toxicol, doi:10.1111/bcpt.12117
Cao, COVID-19: Immunopathology and its implications for therapy, Nat. Rev. Immunol, doi:10.1038/s41577-020-0308-3
Dai, Liu, Liu, Zhou, Li et al., Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak, Cancer Discov, doi:10.1158/2159-8290.CD-20-0422
Drake, Fairfield, Pius, Knight, Norman et al., Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: A matched, prospective cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(21)00104-1
Gomeni, Xu, Gao, Bressolle-Gomeni, Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS CoV-2, J. Pharmacokinet. Pharmacodyn, doi:10.1007/s10928-020-09690-4
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, JAMA, doi:10.1001/jama.2020.5394
Guan, Liang, Zhao, Liang, Chen et al., Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis, Eur. Respir. J, doi:10.1183/13993003.00547-2020
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Huh, Ji, Kang, Hong, Bae et al., Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.12.041
Jung, Choi, You, Kim, Association of Renin-angiotensin-aldosterone System Inhibitors with Coronavirus Disease 2019 (COVID-19)-Related Outcomes in Korea: A Nationwide Population-based Cohort Study, Clin. Infect. Dis, doi:10.1093/cid/ciaa624
Kiani, Scholey, Dahl, Mcmann, Iversen et al., In Vitro Assessment of the Antiviral Activity of Ketotifen, Indomethacin and Naproxen, Alone and in Combination, against SARS-CoV-2, Viruses, doi:10.3390/v13040558
Lighter, Phillips, Hochman, Sterling, Johnson et al., Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission, Clin. Infect. Dis, doi:10.1093/cid/ciaa415
Mehta, Kalra, Nowacki, Anjewierden, Han et al., Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Testing Positive for Coronavirus Disease 2019 (COVID-19), JAMA Cardiol, doi:10.1001/jamacardio.2020.1855
Micallef, Soeiro, Jonville-Béra, Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection, Therapie, doi:10.1016/j.therap.2020.05.003
Park, Lee, You, Kim, Yang, Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19, Sci. Rep, doi:10.1038/s41598-021-84539-5
Prada, Santos, Baião, Costa, Ferreira et al., The Risk of SARS-COV-2 Infection and COVID-19 Severity Associated with The Exposure to Non-Steroidal Anti-Inflammatory Drugs: Systematic Review and Meta-Analysis, J. Clin. Pharmacol, doi:10.1002/jcph.1949
Rodríguez-Morales, Cardona-Ospina, Murillo-Muñoz, Gastroenterologists, Hepatologists, COVID-19 and the Use of Acetaminophen, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2020.04.025
Terrier, Dilly, Pizzorno, Chalupska, Humpolickova et al., Antiviral Properties of the NSAID Drug Naproxen Targeting the Nucleoprotein of SARS-CoV-2 Coronavirus, Molecules, doi:10.3390/molecules26092593
Woo, Lee, Koh, Kim, Han et al., Incidence of cancer after asthma development: 2 independent population-based cohort studies, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2020.04.041
Yon, Lee, Woo, Koh, Jee et al., Exposure to humidifier disinfectants is associated with upper and lower airway diseases, Pediatr. Allergy Immunol, doi:10.1111/pai.13233
Zhao, Huang, Huang, Liu, Shao et al., Prevalence of NSAID use among people with COVID-19 and the association with COVID-19-related outcomes: Systematic review and meta-analysis, Br. J. Clin. Pharmacol, doi:10.1111/bcp.15512
DOI record:
{
"DOI": "10.3390/ijerph20053832",
"ISSN": [
"1660-4601"
],
"URL": "http://dx.doi.org/10.3390/ijerph20053832",
"abstract": "<jats:p>Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) have been widely prescribed to infected patients; however, the safety of them has not been investigated in patients with serious acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Our objective was to evaluate the association between the previous use of acetaminophen or NSAIDs and the clinical outcomes of SARS-CoV-2 infection. A nationwide population-based cohort study was conducted using the Korean Health Insurance Review and Assessment Database through propensity score matching (PSM). A total of 25,739 patients aged 20 years and older who tested for SARS-CoV-2 were included from 1 January 2015 to 15 May 2020. The primary endpoint was a positive result for a SARS-CoV-2 test, and the secondary endpoint was serious clinical outcomes of SARS-CoV-2 infection, such as conventional oxygen therapy, admission to the intensive care unit, need for invasive ventilation care, or death. Of 1058 patients, after propensity score matching, 176 acetaminophen users and 162 NSAIDs users were diagnosed with coronavirus disease 2019. After PSM, 162 paired data sets were generated, and the clinical outcomes of the acetaminophen group were not significantly different from those of the NSAIDs group. This suggests that acetaminophen and NSAIDs can be used safely to control symptoms in patients suspected of having SARS-CoV-2.</jats:p>",
"alternative-id": [
"ijerph20053832"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0186-5834",
"affiliation": [
{
"name": "Department of Orthopaedic Surgery, Nowon Eulji Medical Center, Eulji University, Seoul 01830, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jin-Woo",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4240-4615",
"affiliation": [
{
"name": "Department of Orthopaedic Surgery, CHA Bundang Medical Center, School of Medicine, CHA University, Seongnam-si 13488, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Yoon",
"given": "Siyeong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0136-1372",
"affiliation": [
{
"name": "Department of Orthopaedic Surgery, Nowon Eulji Medical Center, Eulji University, Seoul 01830, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Lee",
"given": "Jongheon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8425-6673",
"affiliation": [
{
"name": "Department of Orthopaedic Surgery, CHA Bundang Medical Center, School of Medicine, CHA University, Seongnam-si 13488, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Lee",
"given": "Soonchul",
"sequence": "additional"
}
],
"container-title": "International Journal of Environmental Research and Public Health",
"container-title-short": "IJERPH",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
2,
22
]
],
"date-time": "2023-02-22T07:57:38Z",
"timestamp": 1677052658000
},
"deposited": {
"date-parts": [
[
2023,
2,
22
]
],
"date-time": "2023-02-22T08:36:33Z",
"timestamp": 1677054993000
},
"funder": [
{
"award": [
"2022R1A2C2005916",
"2022R1A2C2005887"
],
"name": "National Research Foundation of Korea"
}
],
"indexed": {
"date-parts": [
[
2023,
2,
23
]
],
"date-time": "2023-02-23T05:36:37Z",
"timestamp": 1677130597731
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2023,
2,
21
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2023,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
2,
21
]
],
"date-time": "2023-02-21T00:00:00Z",
"timestamp": 1676937600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1660-4601/20/5/3832/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "3832",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
2,
21
]
]
},
"published-online": {
"date-parts": [
[
2023,
2,
21
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41577-020-0308-3",
"article-title": "COVID-19: Immunopathology and its implications for therapy",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_1",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1574",
"journal-title": "JAMA",
"key": "ref_2",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00547-2020",
"article-title": "Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "2000547",
"journal-title": "Eur. Respir. J.",
"key": "ref_3",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa415",
"article-title": "Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission",
"author": "Lighter",
"doi-asserted-by": "crossref",
"first-page": "896",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_4",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1158/2159-8290.CD-20-0422",
"article-title": "Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "783",
"journal-title": "Cancer Discov.",
"key": "ref_5",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3018(20)30111-9",
"article-title": "COVID-19 in patients with HIV: Clinical case series",
"author": "Blanco",
"doi-asserted-by": "crossref",
"first-page": "e314",
"journal-title": "Lancet HIV",
"key": "ref_6",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "ref_7",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(13)60900-9",
"article-title": "Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials",
"author": "Bhala",
"doi-asserted-by": "crossref",
"first-page": "769",
"journal-title": "Lancet",
"key": "ref_8",
"volume": "382",
"year": "2013"
},
{
"DOI": "10.1111/bcpt.12117",
"article-title": "Variability in the response to non-steroidal anti-inflammatory drugs: Mechanisms and perspectives",
"author": "Bruno",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Basic Clin. Pharmacol. Toxicol.",
"key": "ref_9",
"volume": "114",
"year": "2014"
},
{
"DOI": "10.1164/rccm.201802-0229LE",
"article-title": "Nonsteroidal Antiinflammatory Drug Use and Clinical Outcomes of Community-acquired Pneumonia",
"author": "Basille",
"doi-asserted-by": "crossref",
"first-page": "128",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "ref_10",
"volume": "198",
"year": "2018"
},
{
"DOI": "10.1038/s41598-021-84539-5",
"article-title": "Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "5087",
"journal-title": "Sci. Rep.",
"key": "ref_11",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.jaci.2020.04.041",
"article-title": "Incidence of cancer after asthma development: 2 independent population-based cohort studies",
"author": "Woo",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "ref_12",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1111/pai.13233",
"article-title": "Exposure to humidifier disinfectants is associated with upper and lower airway diseases",
"author": "Yon",
"doi-asserted-by": "crossref",
"first-page": "578",
"journal-title": "Pediatr. Allergy Immunol.",
"key": "ref_13",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa624",
"article-title": "Association of Renin-angiotensin-aldosterone System Inhibitors with Coronavirus Disease 2019 (COVID-19)- Related Outcomes in Korea: A Nationwide Population-based Cohort Study",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "2121",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_14",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1001/jamacardio.2020.1855",
"article-title": "Association of Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Testing Positive for Coronavirus Disease 2019 (COVID-19)",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1020",
"journal-title": "JAMA Cardiol.",
"key": "ref_15",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1002/jcph.1949",
"article-title": "The Risk of SARS-COV-2 Infection and COVID-19 Severity Associated with The Exposure to Non-Steroidal Anti-Inflammatory Drugs: Systematic Review and Meta-Analysis",
"author": "Prada",
"doi-asserted-by": "crossref",
"first-page": "1521",
"journal-title": "J. Clin. Pharmacol.",
"key": "ref_16",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1016/S2665-9913(21)00104-1",
"article-title": "Non-steroidal anti-inflammatory drug use and outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK cohort: A matched, prospective cohort study",
"author": "Drake",
"doi-asserted-by": "crossref",
"first-page": "e498",
"journal-title": "Lancet Rheumatol.",
"key": "ref_17",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1111/bcp.15512",
"article-title": "Prevalence of NSAID use among people with COVID-19 and the association with COVID-19-related outcomes: Systematic review and meta-analysis",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "5113",
"journal-title": "Br. J. Clin. Pharmacol.",
"key": "ref_18",
"volume": "88",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.12.041",
"article-title": "Association of prescribed medications with the risk of COVID-19 infection and severity among adults in South Korea",
"author": "Huh",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_19",
"volume": "104",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2022.1063246",
"article-title": "Efficacy and safety profile of corticosteroids and non-steroidal anti-inflammatory drugs in COVID-19 management: A narrative review",
"author": "Amponsah",
"doi-asserted-by": "crossref",
"first-page": "1063246",
"journal-title": "Front. Pharmacol.",
"key": "ref_20",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/molecules26092593",
"doi-asserted-by": "crossref",
"key": "ref_21",
"unstructured": "Terrier, O., Dilly, S., Pizzorno, A., Chalupska, D., Humpolickova, J., Boura, E., Berenbaum, F., Quideau, S., Lina, B., and Feve, B. (2021). Antiviral Properties of the NSAID Drug Naproxen Targeting the Nucleoprotein of SARS-CoV-2 Coronavirus. Molecules, 26."
},
{
"DOI": "10.1007/s10928-020-09690-4",
"article-title": "Model based approach for estimating the dosage regimen of indomethacin a potential antiviral treatment of patients infected with SARS CoV-2",
"author": "Gomeni",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "J. Pharmacokinet. Pharmacodyn.",
"key": "ref_22",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.3390/v13040558",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Kiani, P., Scholey, A., Dahl, T.A., McMann, L., Iversen, J.M., and Verster, J.C. (2021). In Vitro Assessment of the Antiviral Activity of Ketotifen, Indomethacin and Naproxen, Alone and in Combination, against SARS-CoV-2. Viruses, 13."
},
{
"DOI": "10.1016/j.therap.2020.05.003",
"article-title": "Non-steroidal anti-inflammatory drugs, pharmacology, and COVID-19 infection",
"author": "Micallef",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "Therapie",
"key": "ref_24",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.04.025",
"article-title": "Gastroenterologists, Hepatologists, COVID-19 and the Use of Acetaminophen",
"doi-asserted-by": "crossref",
"first-page": "2142",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "ref_25",
"volume": "18",
"year": "2020"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1660-4601/20/5/3832"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health, Toxicology and Mutagenesis",
"Public Health, Environmental and Occupational Health"
],
"subtitle": [],
"title": "Serious Clinical Outcomes of COVID-19 Related to Acetaminophen or NSAIDs from a Nationwide Population-Based Cohort Study",
"type": "journal-article",
"volume": "20"
}