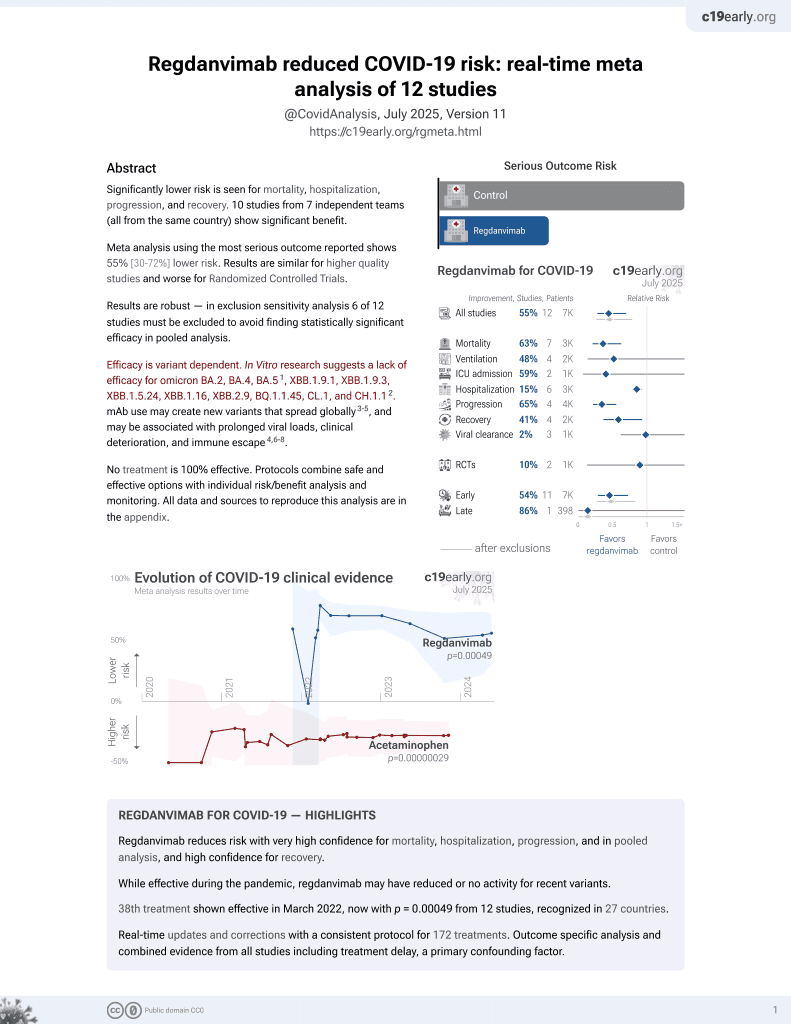
Effectiveness of regdanvimab on mortality in COVID-19 infected patients on hemodialysis
et al., Kidney Research and Clinical Practice, doi:10.23876/j.krcp.23.137, Jan 2024
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
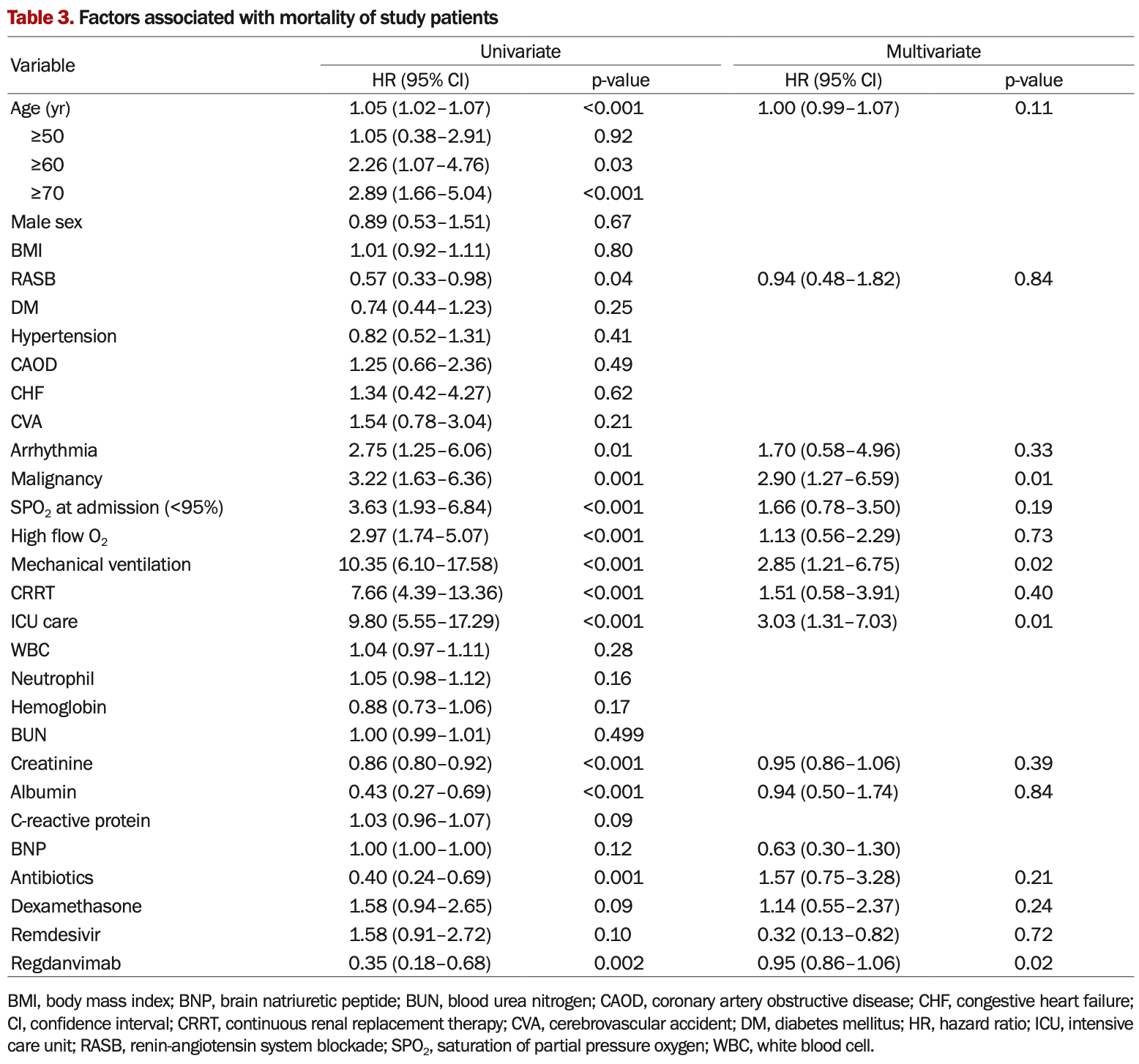
Retrospective 230 hospitalized COVID-19 patients on hemodialysis, reporting lower mortality with regdanvimab treatment. The results are conflicting, with for example the text reporting HR 0.28 for regdanvimab in multivariable analysis, however Table 3 shows HR 0.95. The adjusted analysis is required because the groups are not comparable at baseline, with the control group having a much higher prevalence of severe cases.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
Kee et al., 23 Jan 2024, retrospective, South Korea, peer-reviewed, mean age 67.9, 10 authors, study period 1 December, 2020 - 30 November, 2021.
Contact: km2071@hallym.or.kr, haynepark798@gmail.com.
Effectiveness of regdanvimab on mortality in COVID-19 infected patients on hemodialysis
Kidney Research and Clinical Practice, doi:10.23876/j.krcp.23.137
Background: Although several therapeutic agents have been evaluated for the treatment of coronavirus disease 2019 , there are lack of effective and proven treatments for end-stage renal disease (ESRD). The present study aims to evaluate the effectiveness of regdanvimab on mortality in COVID-19-infected patients on hemodialysis (HD). Methods: We conducted an observational retrospective study in 230 COVID-19-infected patients on HD, of whom 77 (33.5%) were administered regdanvimab alone or in combination with dexamethasone or remdesivir during hospitalization (regdanvimab group) and 153 patients (66.5%) were not (no regdanvimab group). The primary outcome was in-hospital mortality. We compared mortality rates according to the use of regdanvimab and investigated the factors associated with mortality. Results: Fifty-nine deaths occurred during hospitalization, 49 in the no regdanvimab group (32.0%) and 10 in the regdanvimab group (13.0%), and the mortality rate was significantly higher in the no regdanvimab group than that in the regdanvimab group (p = 0.001). Multivariate Cox regression analysis showed that malignancy (p = 0.001), SPO 2 of <95% at admission (p = 0.003), and administration of antibiotics and regdanvimab (p = 0.007 and p = 0.002, respectively) were significantly associated factors with mortality. Conclusion: Regdanvimab administration is beneficial in improving prognosis in hospitalized COVID-19 patients on HD. Considering the vulnerability to infection and high mortality of ESRD patients, regdanvimab may be considered as a therapeutic option in COVID-19 patients on HD.
Conflicts of interest All authors have no conflicts of interest to declare.
Authors' contributions
ORCID Youn Kyung Kee, https://orcid.org/0000-0002-0555-9909 Hayne Cho Park, https://orcid.org/0000-0002-1128-3750 Su Jin Yoon, https://orcid.org/0009-0008-3676-1799 Sungbong Yu, https://orcid.org/0000-0002-1989-6121 Eunsil Ko, https://orcid.org/0000-0001-5959-5545 AJin Cho, https://orcid.org//0000-0001-7097-7026 Do Hyoung Kim, https://orcid.org/0000-0002-8664-8830 Jinseog Kim, https://orcid.org/0000-0003-3172-3212 Young-Ki Lee, https://orcid.org/0000-0003-3464-6144
References
Ariës, Van Den Bergh, Beudel, Clinical course of COVID-19 in the Netherlands: an overview of 2607 patients in hospital during the first wave, Ned Tijdschr Geneeskd
Biswas, Rahaman, Biswas, Haque, Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis, Intervirology, doi:10.1159/000512592
Chae, Choi, Lim, The Effectiveness of the use of regdanvimab (CT-P59) in addition to remdesivir in patients with severe COVID-19: a single center retrospective study, Trop Med Infect Dis, doi:10.3390/tropicalmed7030051
Cheng, Luo, Wang, Kidney disease is associated with in-hospital death of patients with COVID-19, Kidney Int, doi:10.1016/j.kint.2020.03.005
Chupp, Spichler-Moffarah, Søgaard, A phase 2 randomized, double-blind, placebo-controlled trial of oral camostat mesylate for early treatment of COVID-19 outpatients showed shorter illness course and attenuation of loss of smell and taste, medRxiv, doi:10.1101/2022.01.28.22270035
De Meester, Bacquer, Naesens, Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study, J Am Soc Nephrol, doi:10.1681/asn.2020060875
Goicoechea, Cámara, Macías, COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain, Kidney Int, doi:10.1016/j.kint.2020.04.031
Henkens, Raafs, Verdonschot, Age is the main determinant of COVID-19 related in-hospital mortality with minimal impact of pre-existing comorbidities, a retrospective cohort study, BMC Geriatr, doi:10.21203/rs.3.rs-955049/v1
Henry, Lippi, Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection, Int Urol Nephrol, doi:10.1007/s11255-020-02451-9
Jang, Oh, Kim, Clinical effectiveness of regdanvimab treatment for mild-to-moderate COVID-19: a retrospective cohort study, Curr Ther Res Clin Exp, doi:10.1016/j.curtheres.2022.100675
Kato, Chmielewski, Honda, Aspects of immune 10 www.krcp-ksn.org Kidney Res Clin Pract [Epub ahead of print] dysfunction in end-stage renal disease, Clin J Am Soc Nephrol, doi:10.2215/cjn.00950208
Kim, Jang, Hong, Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection, Clin Ther, doi:10.1016/j.clinthera.2021.08.009
Kim, Ryu, Lee, A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat Commun, doi:10.1038/s41467-020-20602-5
Kuriakose, Singh, Pau, Developing treatment guidelines during a pandemic health crisis: lessons learned from COVID-19, Ann Intern Med, doi:10.7326/m21-1647
Langford, So, Raybardhan, Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis, Clin Microbiol Infect, doi:10.1016/j.cmi.2020.07.016
Lee, Lee, Ko, Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease, Front Immunol, doi:10.3389/fimmu.2021.772320
Ly-Cov555 Study, Group, Lundgren, Grund, A neutralizing monoclonal antibody for hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/nejmoa2033130
Park, Lee, Ko, COVID-19-related clinical outcomes among Korean hemodialysis patients, Kidney Res Clin Pract, doi:10.23876/j.krcp.22.023
Portolés, Marques, López-Sánchez, Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak, Nephrol Dial Transplant, doi:10.1093/ndt/gfaa189
Posso, Comas, Román, Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a university hospital in Spain, Arch Bronconeumol (Engl Ed), doi:10.1016/j.arbres.2020.06.012
Rawson, Moore, Castro-Sanchez, COVID-19 and the potential long-term impact on antimicrobial resistance, J Antimicrob Chemother, doi:10.1093/jac/dkaa194
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Saran, Robinson, Abbott, US renal data system 2019 annual data report: epidemiology of kidney disease in the United States, Am J Kidney Dis, doi:10.1053/j.ajkd.2019.09.003
Singh, De, Antiviral agents for the treatment of COVID-19: progress and challenges, Cell Rep Med, doi:10.1016/j.xcrm.2022.100549
Syed, Regdanvimab: first approval, Drugs, doi:10.1007/s40265-021-01626-7
Valeri, Robbins-Juarez, Stevens, Presentation and outcomes of patients with ESKD and COVID-19, J Am Soc Nephrol, doi:10.1681/asn.2020040470
Van De Veerdonk, Bourboulis, Pickkers, A guide to immunotherapy for COVID-19, Nat Med, doi:10.1038/s41591-021-01643-9
Van Duin, Barlow, Nathwani, The impact of the COVID-19 pandemic on antimicrobial resistance: a debate, JAC Antimicrob Resist, doi:10.1093/jacamr/dlaa053
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review, JAMA, doi:10.1001/jama.2020.12839
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
Zuccaro, Celsa, Sambo, Competing-risk analysis of coronavirus disease 2019 in-hospital mortality in a Northern Italian centre from SMAtteo COvid19 REgistry (SMACORE), Sci Rep, doi:10.1038/s41598-020-80679-2
DOI record:
{
"DOI": "10.23876/j.krcp.23.137",
"ISSN": [
"2211-9140"
],
"URL": "http://dx.doi.org/10.23876/j.krcp.23.137",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-05-23"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-09-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published online",
"name": "published",
"order": 2,
"value": "2024-01-23"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0555-9909",
"affiliation": [],
"authenticated-orcid": true,
"family": "Kee",
"given": "Youn Kyung",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1128-3750",
"affiliation": [],
"authenticated-orcid": true,
"family": "Park",
"given": "Hayne Cho",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0008-3676-1799",
"affiliation": [],
"authenticated-orcid": true,
"family": "Yoon",
"given": "Su Jin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1989-6121",
"affiliation": [],
"authenticated-orcid": true,
"family": "Yu",
"given": "Sungbong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5959-5545",
"affiliation": [],
"authenticated-orcid": true,
"family": "Ko",
"given": "Eunsil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cho",
"given": "AJin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8664-8830",
"affiliation": [],
"authenticated-orcid": true,
"family": "Kim",
"given": "Do Hyoung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3172-3212",
"affiliation": [],
"authenticated-orcid": true,
"family": "Kim",
"given": "Jinseog",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3464-6144",
"affiliation": [],
"authenticated-orcid": true,
"family": "Lee",
"given": "Young-Ki",
"sequence": "additional"
},
{
"affiliation": [],
"name": "on behalf of the Korean Society of Nephrology COVID-19 Task Force Team",
"sequence": "additional"
}
],
"container-title": "Kidney Research and Clinical Practice",
"container-title-short": "Kidney Res Clin Pract",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"krcp-ksn.org"
]
},
"created": {
"date-parts": [
[
2024,
1,
23
]
],
"date-time": "2024-01-23T07:05:34Z",
"timestamp": 1705993534000
},
"deposited": {
"date-parts": [
[
2024,
1,
23
]
],
"date-time": "2024-01-23T07:05:34Z",
"timestamp": 1705993534000
},
"indexed": {
"date-parts": [
[
2024,
1,
24
]
],
"date-time": "2024-01-24T00:37:08Z",
"timestamp": 1706056628535
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
1,
23
]
]
},
"language": "en",
"link": [
{
"URL": "http://krcp-ksn.org/upload/pdf/j-krcp-23-137.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "9960",
"original-title": [],
"prefix": "10.23876",
"published": {
"date-parts": [
[
2024,
1,
23
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
23
]
]
},
"publisher": "The Korean Society of Nephrology",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://krcp-ksn.org/journal/view.php?doi=10.23876/j.krcp.23.137"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Urology",
"Nephrology"
],
"subtitle": [],
"title": "Effectiveness of regdanvimab on mortality in COVID-19 infected patients on hemodialysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.23876/crossmark_policy"
}