Clinical Effectiveness of Regdanvimab Treatment for Mild-to-Moderate COVID-19: A Retrospective Cohort Study
et al., Current Therapeutic Research, doi:10.1016/j.curtheres.2022.100675, May 2022
39th treatment shown to reduce risk in
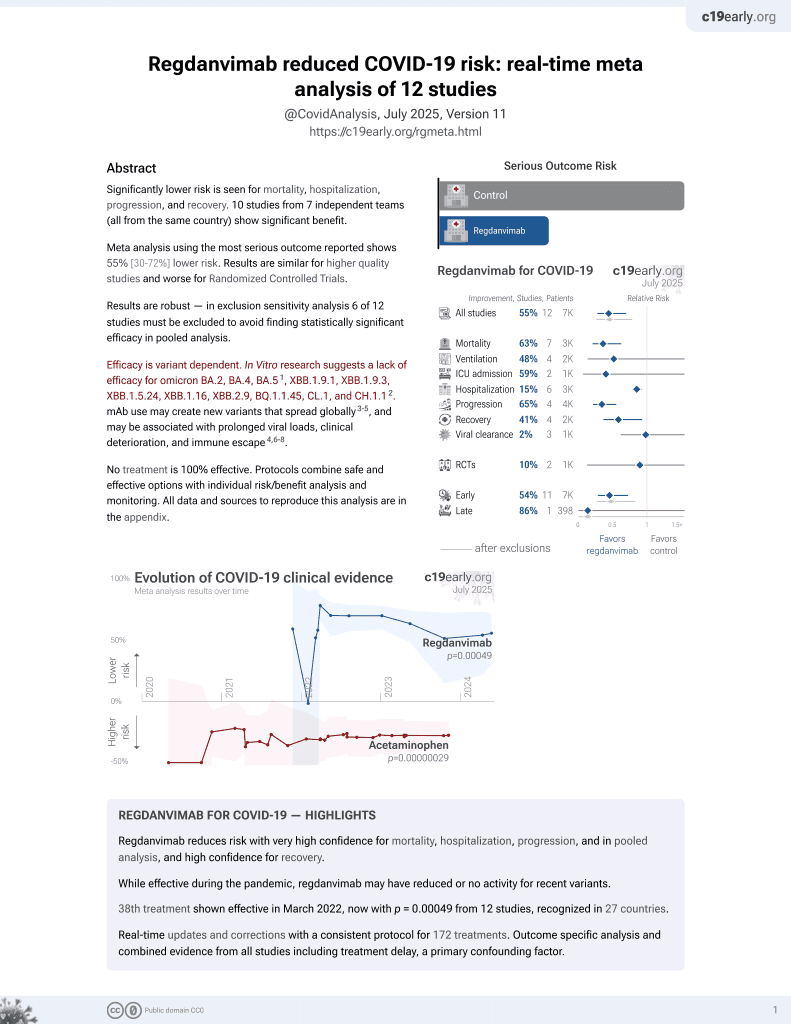
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
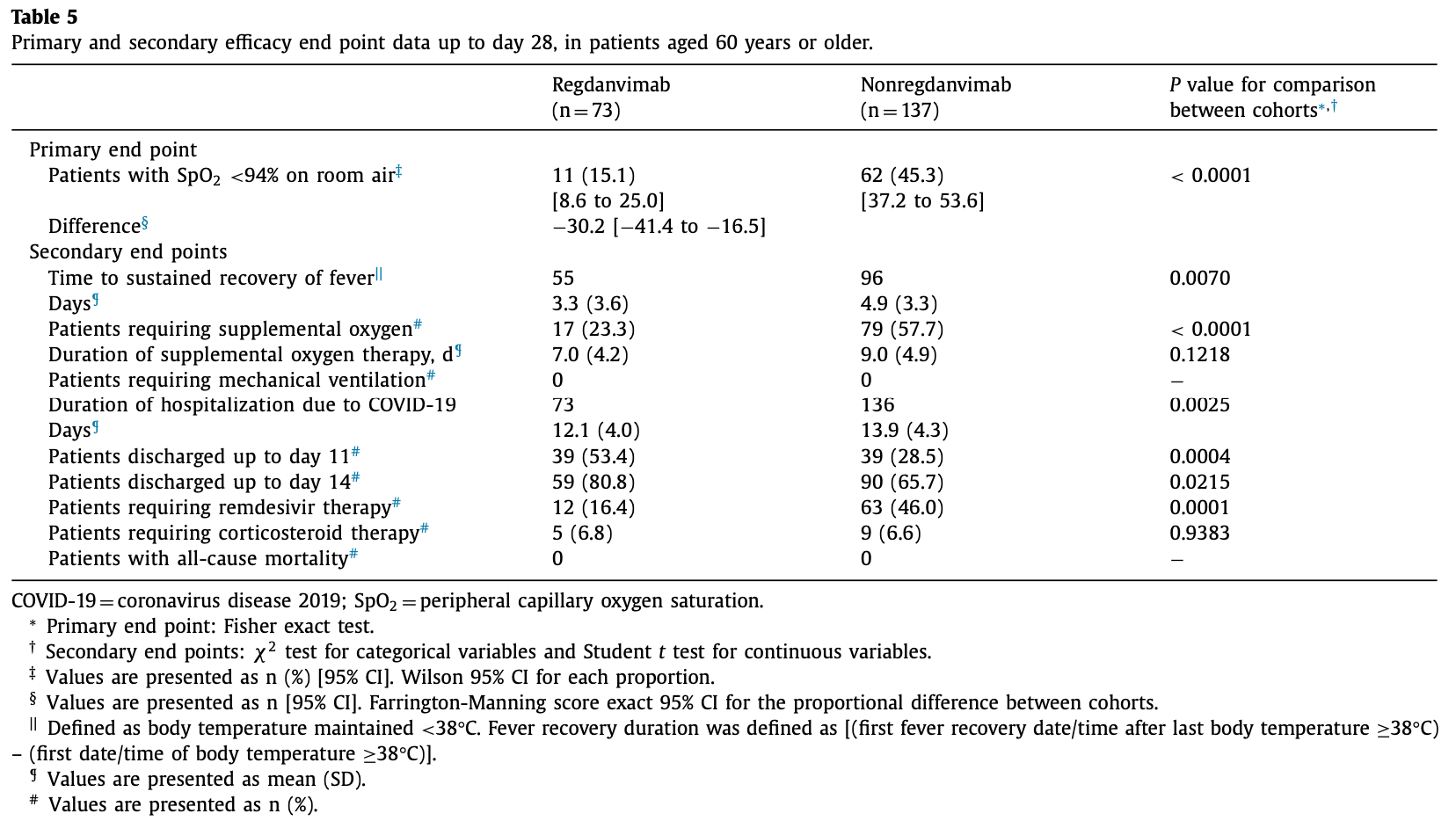
Retrospective 317 hospitalized mild-moderate COVID-19 patients in South Korea showing significantly lower rates of oxygen desaturation (SpO2 <94%) at 28 days (primary outcome) with regdanvimab monoclonal antibody treatment (13%) compared to standard of care (40%). Regdanvimab also showed benefits in time to fever recovery, discharge rates, and supplemental oxygen use.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
|
risk of oxygen therapy, 59.6% lower, RR 0.40, p < 0.001, treatment 17 of 73 (23.3%), control 79 of 137 (57.7%), NNT 2.9.
|
|
risk of no hospital discharge, 44.1% lower, RR 0.56, p = 0.03, treatment 14 of 73 (19.2%), control 47 of 137 (34.3%), NNT 6.6, day 14.
|
|
risk of no hospital discharge, 34.9% lower, RR 0.65, p < 0.001, treatment 34 of 73 (46.6%), control 98 of 137 (71.5%), NNT 4.0, day 11.
|
|
hospitalization time, 12.9% lower, relative time 0.87, p = 0.003, treatment mean 12.1 (±4.0) n=73, control mean 13.9 (±4.3) n=137.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jang et al., 16 May 2022, retrospective, South Korea, peer-reviewed, 3 authors, study period September 2020 - July 2021.
Contact: kjykey@icmc.or.kr.
Clinical Effectiveness of Regdanvimab Treatment for Mild-to-Moderate COVID-19: A Retrospective Cohort Study
Current Therapeutic Research, doi:10.1016/j.curtheres.2022.100675
Background: In a Phase III study, regdanvimab (CT-P59) reduced the risk of hospitalization or death versus placebo in patients with mild-to-moderate coronavirus disease 2019 (COVID-19). Purpose: We performed a retrospective cohort study of patients with COVID-19 to examine the effect of regdanvimab versus standard of care (SoC) on oxygen saturation. Methods: We reviewed patients with mild-to-moderate COVID-19 confirmed by reverse transcriptionpolymerase chain reaction at a single hospital in the Republic of Korea. The primary efficacy end point was the proportion of patients deteriorating with peripheral capillary oxygen saturation < 94% on room air up to day 28. Results: A total of 127 patients were treated for COVID-19 with regdanvimab, 190 with SoC. The proportion of patients deteriorating with peripheral capillary oxygen saturation < 94% on room air up to day 28 was 13.4% with regdanvimab and 39.5% with SoC ( P < 0.0 0 01); median time (range) until sustained recovery of fever was 2.0 (0.2-14.8) and 4.2 (0.1-17.1) days, respectively. Supplemental oxygen was required by 23.6% of patients with regdanvimab and 52.1% with SoC ( P < 0.0 0 01) for a mean of 6.3 and 8.7 days, respectively ( P = 0.0113); no patients needed mechanical ventilation. Compared with SoC, hospitalization was shorter with regdanvimab (mean = 11.1 vs 13.6 days; 63.8% vs 31.6% discharged within 11 days; both P values < 0.0 0 01). Fewer regdanvimab-treated patients required remdesivir (14.2% vs 43.2%; P < 0.0 0 01). There were no deaths. Two patients had adverse reactions with regdanvimab. Conclusions: This real-world study indicates that regdanvimab can prevent deterioration in patients with mild-to-moderate COVID-19. (
Conflicts of Interest Statement The authors have indicated that they have no conflicts of interest regarding the content of this article.
References
Alam, Mahmud, Aggarwal, Clinical impact of the early use of monoclonal antibody LY-CoV555 (bamlanivimab) on mortality and hospitalization among elderly nursing home patients: a multicenter retrospective study, Cureus
Baraniuk, Where are we with drug treatments for covid-19?, BMJ
Berlin, Gulick, Martinez, Severe Covid-19, N Engl J Med
Bierle, Ganesh, Wilker, Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high--risk patients with mild-moderate COVID-19, J Prim Care Community Health
Brouqui, Amrane, Million, Asymptomatic hypoxia in COVID-19 is associated with poor outcome, Int J Infect Dis
Chen, Feng, Xu, Patterns of deterioration in moderate patients with COVID-19 from Jan 2020 to Mar 2020: a multi-center, retrospective cohort study in China, Front Med
Corti, Purcell, Snell, Veesler, Tackling COVID-19 with neutralizing monoclonal antibodies, Cell
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Gupta, Marks, Samuels, Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study, Eur Respir J
Kim, Jang, Hong, Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection, Clin Ther
Kumar, Wu, Stosor, Real-world experience of bamlanivimab for COVID-19: a case-control study, Clin Infect Dis
Marston, Paules, Fauci, Monoclonal antibodies for emerging infectious diseases -borrowing from history, N Engl J Med
Rechtman, Curtin, Navarro, Nirenberg, Horton, Vital signs assessed in initial clinical encounters predict COVID-19 mortality in an NYC hospital system, Sci Rep
Ryu, Kang, Noh, The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2, Biochem Biophys Res Commun
Ryu, Song, Kim, Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant, Biochem Biophys Res Commun
Sandulsecu, Therapeutic effect of regdanvimab in patients with mild to moderate COVID-19: day 28 results from a multi-centre, randomised, controlled pivotal trial
Sanitária -Anvisa, Anvisa autoriza uso emergencial de novo medicamento para Covid-19
Syed, Regdanvimab: First Approval, Drugs
Venkatesan, Repurposing drugs for treatment of COVID-19, Lancet Respir Med
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Wouters, Shadlen, Salcher-Konrad, Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment, Lancet
Zhai, Luo, Zheng, Moderate vs. mild cases of overseas-imported COVID-19 in Beijing: a retrospective cohort study, Sci Rep
Zhang, Wang, Zhao, The clinical characteristics and prognosis factors of mild-moderate patients with COVID-19 in a mobile cabin hospital: a retrospective, single-center study, Front Public Health
DOI record:
{
"DOI": "10.1016/j.curtheres.2022.100675",
"ISSN": [
"0011-393X"
],
"URL": "http://dx.doi.org/10.1016/j.curtheres.2022.100675",
"alternative-id": [
"S0011393X22000145"
],
"article-number": "100675",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Clinical Effectiveness of Regdanvimab Treatment for Mild-to-Moderate COVID-19: A Retrospective Cohort Study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Current Therapeutic Research"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.curtheres.2022.100675"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Jang",
"given": "Young Rock",
"sequence": "first"
},
{
"affiliation": [],
"family": "Oh",
"given": "Yoon Ju",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4306-1597",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jin Yong",
"sequence": "additional"
}
],
"container-title": "Current Therapeutic Research",
"container-title-short": "Current Therapeutic Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
16
]
],
"date-time": "2022-05-16T22:25:09Z",
"timestamp": 1652739909000
},
"deposited": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T06:48:04Z",
"timestamp": 1673592484000
},
"funder": [
{
"DOI": "10.13039/100018592",
"doi-asserted-by": "publisher",
"name": "Celltrion"
}
],
"indexed": {
"date-parts": [
[
2023,
10,
25
]
],
"date-time": "2023-10-25T05:53:09Z",
"timestamp": 1698213189692
},
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2022
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 132,
"start": {
"date-parts": [
[
2022,
5,
13
]
],
"date-time": "2022-05-13T00:00:00Z",
"timestamp": 1652400000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0011393X22000145?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0011393X22000145?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100675",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.curtheres.2022.100675_bib0001",
"unstructured": "World Health Organization. WHO coronavirus disease (COVID-19) dashboard; 2021. Available from: https://covid19.who.int/. Accessed November 15, 2021."
},
{
"DOI": "10.1016/S0140-6736(21)00306-8",
"article-title": "Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment",
"author": "Wouters",
"doi-asserted-by": "crossref",
"first-page": "1023",
"journal-title": "Lancet",
"key": "10.1016/j.curtheres.2022.100675_bib0002",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n1109",
"article-title": "Where are we with drug treatments for covid-19?",
"author": "Baraniuk",
"doi-asserted-by": "crossref",
"first-page": "n1109",
"journal-title": "BMJ",
"key": "10.1016/j.curtheres.2022.100675_bib0003",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00270-8",
"article-title": "Repurposing drugs for treatment of COVID-19",
"author": "Venkatesan",
"doi-asserted-by": "crossref",
"first-page": "e63",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.curtheres.2022.100675_bib0004",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMp1802256",
"article-title": "Monoclonal antibodies for emerging infectious diseases - borrowing from history",
"author": "Marston",
"doi-asserted-by": "crossref",
"first-page": "1469",
"journal-title": "N Engl J Med",
"key": "10.1016/j.curtheres.2022.100675_bib0005",
"volume": "378",
"year": "2018"
},
{
"DOI": "10.1016/j.cell.2021.05.005",
"article-title": "Tackling COVID-19 with neutralizing monoclonal antibodies",
"author": "Corti",
"doi-asserted-by": "crossref",
"first-page": "3086",
"journal-title": "Cell",
"key": "10.1016/j.curtheres.2022.100675_bib0006",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2020.567296",
"article-title": "Patterns of deterioration in moderate patients with COVID-19 from Jan 2020 to Mar 2020: a multi-center, retrospective cohort study in China",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "Front Med",
"key": "10.1016/j.curtheres.2022.100675_bib0007",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "10.1016/j.curtheres.2022.100675_bib0008",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/j.curtheres.2022.100675_bib0009",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.clinthera.2021.08.009",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "1706",
"journal-title": "Clin Ther",
"key": "10.1016/j.curtheres.2022.100675_bib0010",
"volume": "43",
"year": "2021"
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0011",
"unstructured": "Sandulsecu O. Therapeutic effect of regdanvimab in patients with mild to moderate COVID-19: day 28 results from a multi-centre, randomised, controlled pivotal trial. Presented at: 31st European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); July 9–12, 2021; Online. Presentation 4650."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0012",
"unstructured": "The Korea Economic Daily. Celltrion's COVID-19 treatment reduces incidence of severity by 70%; 2021. Available from: https://www.kedglobal.com/newsView/ked202107130018. Accessed September 29, 2021."
},
{
"DOI": "10.1007/s40265-021-01626-7",
"article-title": "Regdanvimab: First Approval",
"author": "Syed",
"doi-asserted-by": "crossref",
"first-page": "2133",
"journal-title": "Drugs",
"key": "10.1016/j.curtheres.2022.100675_bib0013",
"volume": "81",
"year": "2021"
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0014",
"unstructured": "Agência Nacional de Vigilância Sanitária - Anvisa. Anvisa autoriza uso emergencial de novo medicamento para Covid-19; 2021. Available from: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/anvisa-autoriza-uso-emergencial-de-novo-medicamento-para-covid-19. Accessed September 6, 2021."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0015",
"unstructured": "European Medicines Agency. COVID-19: EMA recommends authorisation of two monoclonal antibody medicines 2021. Available from: https://www.ema.europa.eu/en/news/covid-19-ema-recommends-authorisation-two-monoclonal-antibody-medicines. Accessed November 11, 2021."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0016",
"unstructured": "Australian Government Department of Health Therapeutic Goods Administration. TGA provisional approval of Celltrion Healthcare Australia Pty Ltd COVID-19 treatment, regdanvimab (REGKIRONA); 2021. Available from: https://www.tga.gov.au/media-release/tga-provisional-approval-celltrion-healthcare-australia-pty-ltd-covid-19-treatment-regdanvimab-regkirona. Accessed December 13, 2021."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0017",
"unstructured": "Republic of Korea: Korea Disease Control and Prevention Agency. The current status of COVID-19 treatment (February 4, 2022) [Korean]; 2022. Available from: http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6352&board_id=312&contSeq=6352. Accessed February 14, 2022."
},
{
"DOI": "10.1183/13993003.03498-2020",
"article-title": "Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study",
"author": "Gupta",
"doi-asserted-by": "crossref",
"journal-title": "Eur Respir J",
"key": "10.1016/j.curtheres.2022.100675_bib0018",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1038/s41598-020-78392-1",
"article-title": "Vital signs assessed in initial clinical encounters predict COVID-19 mortality in an NYC hospital system",
"author": "Rechtman",
"doi-asserted-by": "crossref",
"first-page": "21545",
"journal-title": "Sci Rep",
"key": "10.1016/j.curtheres.2022.100675_bib0019",
"volume": "10",
"year": "2020"
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0020",
"unstructured": "Republic of Korea: Central Disaster Management Headquarters and Central Disease Control Headquarters. Coronavirus disease-19: patient treatment and management; 2021. Available from: http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=112&dataGubun=&ncvContSeq=&contSeq=&board_id=&gubun=. Accessed October 1, 2021."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0021",
"unstructured": "World Health Organization. Living guidance for clinical management of COVID-19. November 23, 2021; 2021. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2. Accessed February 04, 2022."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0022",
"unstructured": "Korea Legislation Research Institute, Korean Law Translation Center. Statutes of the Republic of Korea, Bioethics and Safety Act; 2014. Available from: https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=33442&type=part&key=36. Accessed June 11, 2021."
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0023",
"unstructured": "National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines; 2021. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed June 14, 2021."
},
{
"DOI": "10.1016/j.ijid.2020.10.067",
"article-title": "Asymptomatic hypoxia in COVID-19 is associated with poor outcome",
"author": "Brouqui",
"doi-asserted-by": "crossref",
"first-page": "233",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.curtheres.2022.100675_bib0024",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1056/NEJMcp2009575",
"article-title": "Severe Covid-19",
"author": "Berlin",
"doi-asserted-by": "crossref",
"first-page": "2451",
"journal-title": "N Engl J Med.",
"key": "10.1016/j.curtheres.2022.100675_bib0025",
"volume": "383",
"year": "2020"
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0026",
"unstructured": "World Health Organization. Interim guidance for member states - on the use of pulse oximetry in monitoring Covid-19 patients under home-based isolation and care 2021. Available from: https://www.afro.who.int/sites/default/files/Covid-19/Techinical%20documents/GUIDELINES%20FOR%20THE%20USE%20OF%20PULSE%20OXIMETRY%20IN%20MONITORING%20COVID-19%20PATIENTS%20IN%20HBIC.pdf. Accessed September 29, 2021."
},
{
"DOI": "10.3389/fpubh.2020.00264",
"article-title": "The clinical characteristics and prognosis factors of mild-moderate patients with COVID-19 in a mobile cabin hospital: a retrospective, single-center study",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "264",
"journal-title": "Front Public Health",
"key": "10.1016/j.curtheres.2022.100675_bib0027",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-85869-0",
"article-title": "Moderate vs. mild cases of overseas-imported COVID-19 in Beijing: a retrospective cohort study",
"author": "Zhai",
"doi-asserted-by": "crossref",
"first-page": "6483",
"journal-title": "Sci Rep",
"key": "10.1016/j.curtheres.2022.100675_bib0028",
"volume": "11",
"year": "2021"
},
{
"article-title": "Clinical impact of the early use of monoclonal antibody LY-CoV555 (bamlanivimab) on mortality and hospitalization among elderly nursing home patients: a multicenter retrospective study",
"author": "Alam",
"first-page": "e14933",
"journal-title": "Cureus",
"key": "10.1016/j.curtheres.2022.100675_bib0029",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciab305",
"article-title": "Real-world experience of bamlanivimab for COVID-19: a case-control study",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "24",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.curtheres.2022.100675_bib0030",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1177/21501327211019282",
"article-title": "Influence of social and cultural factors on the decision to consent for monoclonal antibody treatment among high-risk patients with mild-moderate COVID-19",
"author": "Bierle",
"doi-asserted-by": "crossref",
"journal-title": "J Prim Care Community Health",
"key": "10.1016/j.curtheres.2022.100675_bib0031",
"volume": "12",
"year": "2021"
},
{
"key": "10.1016/j.curtheres.2022.100675_bib0032",
"unstructured": "European Medicines Agency. Regdanvimab assessment report; 2021. Available from: https://www.ema.europa.eu/en/documents/referral/regdanvimab-treatment-covid-19-celltrion-covid-19-article-53-procedure-assessment-report_en.pdf. Accessed September 30, 2021."
},
{
"DOI": "10.1016/j.bbrc.2021.06.016",
"article-title": "Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "135",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.1016/j.curtheres.2022.100675_bib0033",
"volume": "566",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2021.09.023",
"article-title": "The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Biochem Biophys Res Commun",
"key": "10.1016/j.curtheres.2022.100675_bib0034",
"volume": "578",
"year": "2021"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0011393X22000145"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Clinical Effectiveness of Regdanvimab Treatment for Mild-to-Moderate COVID-19: A Retrospective Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "96"
}