The Effectiveness of the Use of Regdanvimab (CT-P59) in Addition to Remdesivir in Patients with Severe COVID-19: A Single Center Retrospective Study
et al., Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed7030051, Mar 2022
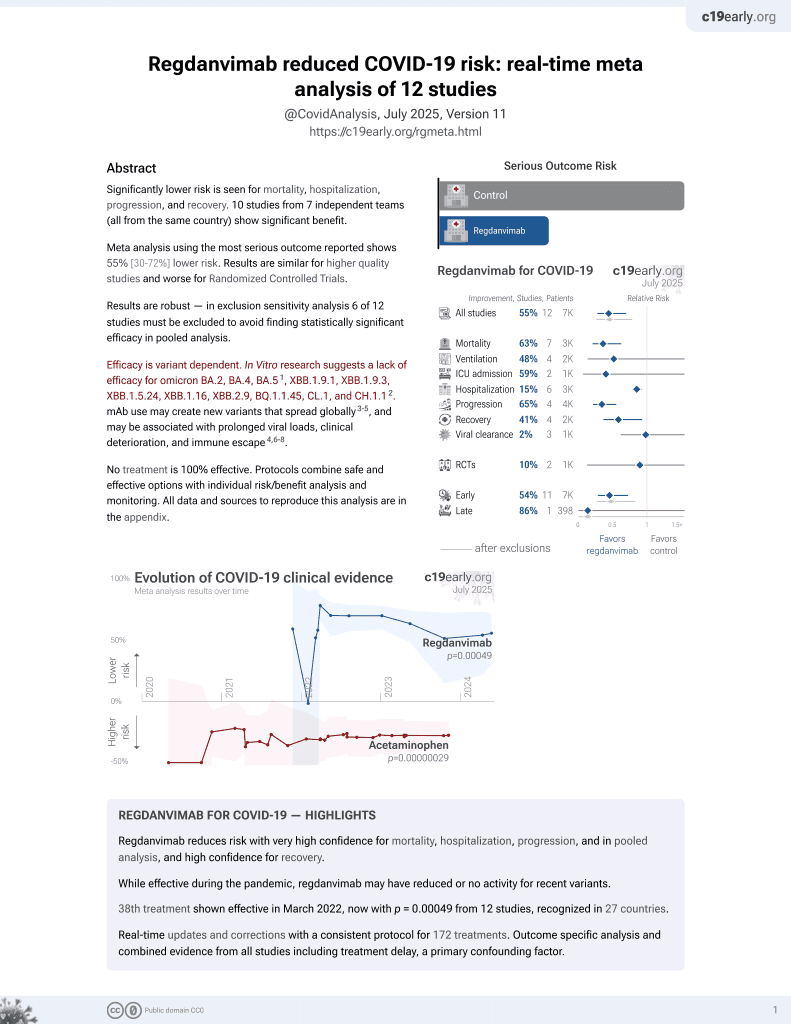
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
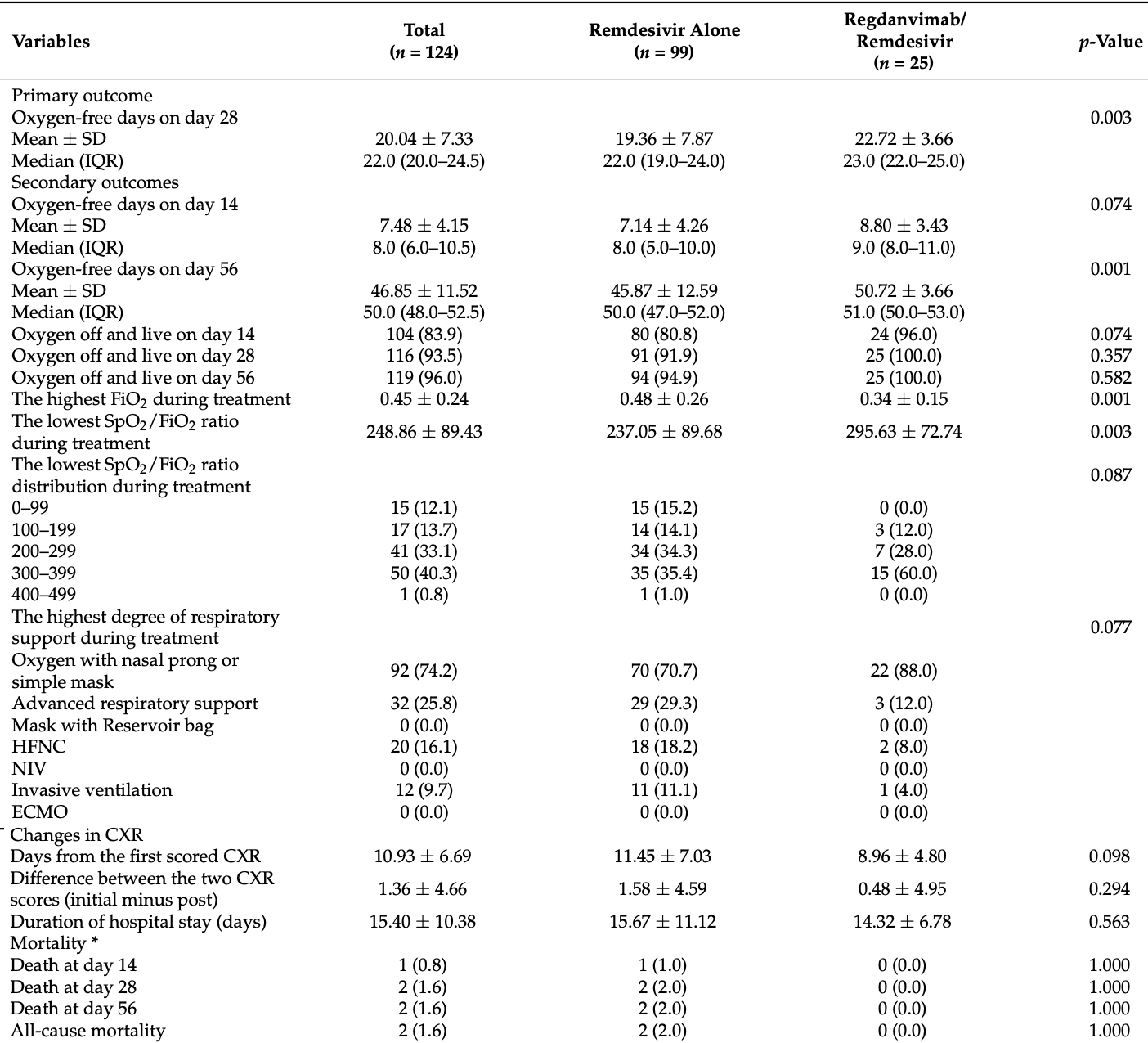
Retrospective 124 hospitalized severe COVID-19 patients receiving oxygen and remdesivir treatment in South Korea. A subgroup of 25 patients also received the monoclonal antibody regdanvimab prior to remdesivir. The regdanvimab subgroup had significantly more oxygen-free days (primary outcome), higher oxygen saturation, less advanced respiratory support, and shorter oxygen supplementation duration compared to the remdesivir alone group.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
Although the 71% lower mortality is not statistically significant, it is consistent with the significant 63% lower mortality [37‑79%] from meta-analysis of the 7 mortality results to date.
|
risk of death, 71.5% lower, RR 0.29, p = 1.00, treatment 0 of 25 (0.0%), control 2 of 99 (2.0%), NNT 49, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 64.0% lower, RR 0.36, p = 0.46, treatment 1 of 25 (4.0%), control 11 of 99 (11.1%), NNT 14.
|
|
hospitalization time, 8.6% lower, relative time 0.91, p = 0.56, treatment mean 14.32 (±6.78) n=25, control mean 15.67 (±11.12) n=99.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chae et al., 18 Mar 2022, retrospective, South Korea, peer-reviewed, mean age 57.6, 14 authors, study period 1 March, 2021 - 11 May, 2021, average treatment delay 3.68 days.
Contact: becareful123@uuh.ulsan.kr (corresponding author), margiela07@naver.com, 0735483@uuh.ulsan.kr, 0733808@uuh.ulsan.kr, docra@docra.pe.kr, yjjegal@uuh.ulsan.kr, jjahn@uuh.ulsan.kr, jjb@uuh.ulsan.kr, soyeoun.lim.xr@uuh.ulsan.kr, gamju@uuh.ulsan.kr, 0735496@uuh.ulsan.kr, 0735495@uuh.ulsan.kr, 0735779@uuh.ulsan.kr, tleepulalg@uuh.ulsan.kr.
The Effectiveness of the Use of Regdanvimab (CT-P59) in Addition to Remdesivir in Patients with Severe COVID-19: A Single Center Retrospective Study
Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed7030051
Introduction: Coronavirus disease 2019 (COVID-19) still has a high mortality rate when it is severe. Regdanvimab (CT-P59), a neutralizing monoclonal antibody that has been proven effective against mild to moderate COVID-19, may be effective against severe COVID-19. This study was conducted to determine the effectiveness of the combined use of remdesivir and regdanvimab in patients with severe COVID-19. Methods: From March to early May 2021, 124 patients with severe COVID-19 were admitted to Ulsan University Hospital (Ulsan, Korea) and received oxygen therapy and remdesivir. Among them, 25 were also administered regdanvimab before remdesivir. We retrospectively compared the clinical outcomes between the remdesivir alone group [n = 99 (79.8%)] and the regdanvimab/remdesivir group [n = 25 (20.2%)]. Results: The oxygenfree days on day 28 (primary outcome) were significantly higher in the regdanvimab/remdesivir group [mean ± SD: 19.36 ± 7.87 vs. 22.72 ± 3.66, p = 0.003]. The oxygen-free days was also independently associated with use of regdanvimab in the multivariate analysis, after adjusting for initial pulse oximetric saturation (SpO 2 )/fraction of inspired oxygen (FiO 2 ) ratio (severity index). Further, in the regdanvimab/remdesivir group, the lowest SpO 2 /FiO 2 ratio during treatment was significantly higher (mean ± SD: 237.05 ± 89.68 vs. 295.63 ± 72.74, p = 0.003), and the Kaplan-Meier estimates of oxygen supplementation days in surviving patients (on day 28) were significantly shorter [mean ± SD: 8.24 ± 7.43 vs. 5.28 ± 3.66, p = 0.024]. Conclusions: In patients with severe COVID-19, clinical outcomes can be improved by administering regdanvimab, in addition to remdesivir.
Author Contributions: Conceptualization, G.C., A.C., W.K. and T.L.; Methodology, A.C., E.P. and T.L.; Formal Analysis, A.C., E.P. and T.L.; Data Curation, A.C. and T.L.; Writing-Original Draft Preparation, T.L.; Writing-Review & Editing, G.C., S.L., S.P., S.L., Y.A., J.K., S.R., Y.J., J.A. and J.J. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare no conflict of interest.
References
Activ, Ly-Cov555, Group; Lundgren, Grund, Barkauskas et al., A neutralizing monoclonal antibody for hospitalized patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2033130
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of COVID-19-final report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Borghesi, Maroldi, COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression, Radiol. Med, doi:10.1007/s11547-020-01200-3
Choi, Hong, Kim, The association between mortality and the oxygen saturation and fraction of inhaled oxygen in patients requiring oxygen therapy due to COVID-19-associated pneumonia, Tuberc. Respir. Dis, doi:10.4046/trd.2020.0126
Eom, Ison, Streinu-Cercel, Săndulescu, Preotescu et al., mild-to-moderate SARS-CoV-2 infection, doi:10.21203/rs.3.rs-296518/v1
Group; Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Kim, Jang, Hong, Jung, Park et al., Virologic Efficacy, and Pharmacokinetics of CT-P59, a Neutralizing Monoclonal Antibody Against SARS-CoV-2 Spike Receptor-Binding Protein: Two Randomized, Placebo-Controlled, Phase I Studies in Healthy Individuals and Patients With Mild SARS-CoV-2 Infection, Clin. Ther, doi:10.1016/j.clinthera.2021.08.009
Kim, Kim, Huh, Choi, Kim et al., Korean Society of Infectious Diseases/National Evidence-based Healthcare Collaborating Agency recommendations for anti-SARS-CoV-2 monoclonal antibody treatment of patients with COVID-19, Infect. Chemother, doi:10.3947/ic.2021.0304
Kim, Ryu, Lee, Kim, Seo et al., A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat. Commun, doi:10.1038/s41467-020-20602-5
Lee, Lee, Ko, Hyun, Kim et al., Effectiveness of Regdanvimab Treatment in High-Risk COVID-19 Patients to Prevent Progression to Severe Disease, Front. Immunol, doi:10.3389/fimmu.2021.772320
Lee, Lee, Lee, Kim, Lee et al., Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: A propensity score-matched retrospective cohort study, Int. Immunopharmacol, doi:10.1016/j.intimp.2022.108570
Lee, Lim, Hong, Park, Kim et al., The primary report of clinical data analysis on the COVID-19 in the Republic of Korea, Public Health Wkly. Rep
Lee, Mcdonald, Butler-Laporte, Harrison, Cheng et al., Remdesivir and systemic corticosteroids for the treatment of COVID-19: A Bayesian re-analysis, Int. J. Infect. Dis, doi:10.1016/j.ijid.2021.01.065
Olivas-Martínez, Cárdenas-Fragoso, Jiménez, Lozano-Cruz, Ortiz-Brizuela et al., In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation, PLoS ONE, doi:10.1371/journal.pone.0245772
Park, Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19), Clin. Exp. Pediatr, doi:10.3345/cep.2020.00493
Regeneron, REGEN-COV™ (Casirivimab and Imdevimab) Phase 3 Recovery Trial Meets Primary Outcome, Improving Survival in Hospitalized COVID-19 Patients Lacking an Immune Response to SARS-COV-2
Rice, Wheeler, Bernard, Hayden, Schoenfeld et al., and Blood Institute ARDS Network. Comparison of the SpO 2 /FIO 2 ratio and the PaO 2 /FIO 2 ratio in patients with acute lung injury or ARDS, Chest, doi:10.1378/chest.07-0617
Ryu, Kang, Noh, Woo, Lee et al., The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2021.09.023
Sheikh, Mcmenamin, Taylor, Robertson, Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness, Lancet, doi:10.1016/S0140-6736(21)01358-1
Simonis, Serpa Neto, Binnekade, Braber, Bruin et al., Effect of a low vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: A randomized clinical trial, JAMA, doi:10.1001/jama.2018.14280
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat. Rev. Immunol, doi:10.1038/s41577-021-00542-x
Ulrich, Troxel, Carmody, Eapen, Bäcker et al., Treating COVID-19 with hydroxychloroquine (TEACH): A multicenter, double-blind randomized controlled trial in hospitalized patients, Open Forum Infect. Dis, doi:10.1093/ofid/ofaa446
Waghmare, Xie, Kimball, Yi, Özkök et al., Supplemental oxygen-free days in hematopoietic cell transplant recipients with respiratory syncytial virus, J. Infect. Dis, doi:10.1093/infdis/jix390
Wang, Wang, Identification of risk factors for in-hospital death of COVID-19 pneumonia -lessions from the early outbreak, BMC Infect. Dis, doi:10.1186/s12879-021-05814-4
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
DOI record:
{
"DOI": "10.3390/tropicalmed7030051",
"ISSN": [
"2414-6366"
],
"URL": "http://dx.doi.org/10.3390/tropicalmed7030051",
"abstract": "<jats:p>Introduction: Coronavirus disease 2019 (COVID-19) still has a high mortality rate when it is severe. Regdanvimab (CT-P59), a neutralizing monoclonal antibody that has been proven effective against mild to moderate COVID-19, may be effective against severe COVID-19. This study was conducted to determine the effectiveness of the combined use of remdesivir and regdanvimab in patients with severe COVID-19. Methods: From March to early May 2021, 124 patients with severe COVID-19 were admitted to Ulsan University Hospital (Ulsan, Korea) and received oxygen therapy and remdesivir. Among them, 25 were also administered regdanvimab before remdesivir. We retrospectively compared the clinical outcomes between the remdesivir alone group [n = 99 (79.8%)] and the regdanvimab/remdesivir group [n = 25 (20.2%)]. Results: The oxygen-free days on day 28 (primary outcome) were significantly higher in the regdanvimab/remdesivir group [mean ± SD: 19.36 ± 7.87 vs. 22.72 ± 3.66, p = 0.003]. The oxygen-free days was also independently associated with use of regdanvimab in the multivariate analysis, after adjusting for initial pulse oximetric saturation (SpO2)/fraction of inspired oxygen (FiO2) ratio (severity index). Further, in the regdanvimab/remdesivir group, the lowest SpO2/FiO2 ratio during treatment was significantly higher (mean ± SD: 237.05 ± 89.68 vs. 295.63 ± 72.74, p = 0.003), and the Kaplan-Meier estimates of oxygen supplementation days in surviving patients (on day 28) were significantly shorter [mean ± SD: 8.24 ± 7.43 vs. 5.28 ± 3.66, p = 0.024]. Conclusions: In patients with severe COVID-19, clinical outcomes can be improved by administering regdanvimab, in addition to remdesivir.</jats:p>",
"alternative-id": [
"tropicalmed7030051"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7480-2634",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chae",
"given": "Ganghee",
"sequence": "first"
},
{
"affiliation": [],
"family": "Choi",
"given": "Aram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Soyeoun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Sooneun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Seungjun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahn",
"given": "Youngick",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2229-2388",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jinhyoung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2458-8414",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ra",
"given": "Seungwon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jegal",
"given": "Yangjin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahn",
"given": "Jongjoon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7967-5518",
"affiliation": [],
"authenticated-orcid": false,
"family": "Park",
"given": "Eunji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jun",
"given": "Jaebum",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8803-0049",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kwon",
"given": "Woonjung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Taehoon",
"sequence": "additional"
}
],
"container-title": "Tropical Medicine and Infectious Disease",
"container-title-short": "TropicalMed",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T01:37:17Z",
"timestamp": 1647826637000
},
"deposited": {
"date-parts": [
[
2022,
3,
21
]
],
"date-time": "2022-03-21T02:35:38Z",
"timestamp": 1647830138000
},
"indexed": {
"date-parts": [
[
2023,
8,
21
]
],
"date-time": "2023-08-21T21:12:42Z",
"timestamp": 1692652362426
},
"is-referenced-by-count": 3,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3,
18
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
18
]
],
"date-time": "2022-03-18T00:00:00Z",
"timestamp": 1647561600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2414-6366/7/3/51/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "51",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
18
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
18
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3345/cep.2020.00493",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"key": "ref2"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1016/j.ijid.2021.01.065",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"article-title": "The primary report of clinical data analysis on the COVID-19 in the Republic of Korea",
"author": "Lee",
"first-page": "2054",
"journal-title": "Public Health Wkly. Rep.",
"key": "ref7",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0245772",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1186/s12879-021-05814-4",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.clinthera.2021.08.009",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.21203/rs.3.rs-296518/v1",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.bbrc.2021.09.023",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"key": "ref14"
},
{
"key": "ref15"
},
{
"DOI": "10.1378/chest.07-0617",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1007/s11547-020-01200-3",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.4046/trd.2020.0126",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1001/jama.2018.14280",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1093/infdis/jix390",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1093/ofid/ofaa446",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1016/S0140-6736(21)01358-1",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1038/s41577-021-00542-x",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3947/ic.2021.0304",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.intimp.2022.108570",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.3389/fimmu.2021.772320",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1056/NEJMoa2033130",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"key": "ref28",
"unstructured": "REGEN-COV™ (Casirivimab and Imdevimab) Phase 3 Recovery Trial Meets Primary Outcome, Improving Survival in Hospitalized COVID-19 Patients Lacking an Immune Response to SARS-COV-2https://investor.regeneron.com/news-releases/news-release-details/regen-covtm-casirivimab-and-imdevimab-phase-3-recovery-trial"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2414-6366/7/3/51"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Immunology and Microbiology"
],
"subtitle": [],
"title": "The Effectiveness of the Use of Regdanvimab (CT-P59) in Addition to Remdesivir in Patients with Severe COVID-19: A Single Center Retrospective Study",
"type": "journal-article",
"volume": "7"
}