Reszinate—A Phase 1/2 Randomized Clinical Trial of Zinc and Resveratrol Utilizing Home Patient-Obtained Nasal and Saliva Viral Sampling
et al., Frontiers in Drug Discovery, doi:10.3389/fddsv.2022.91012, Reszinate, NCT04542993, Oct 2021
Zinc for COVID-19
2nd treatment shown to reduce risk in
July 2020, now with p = 0.00000019 from 42 studies, recognized in 23 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
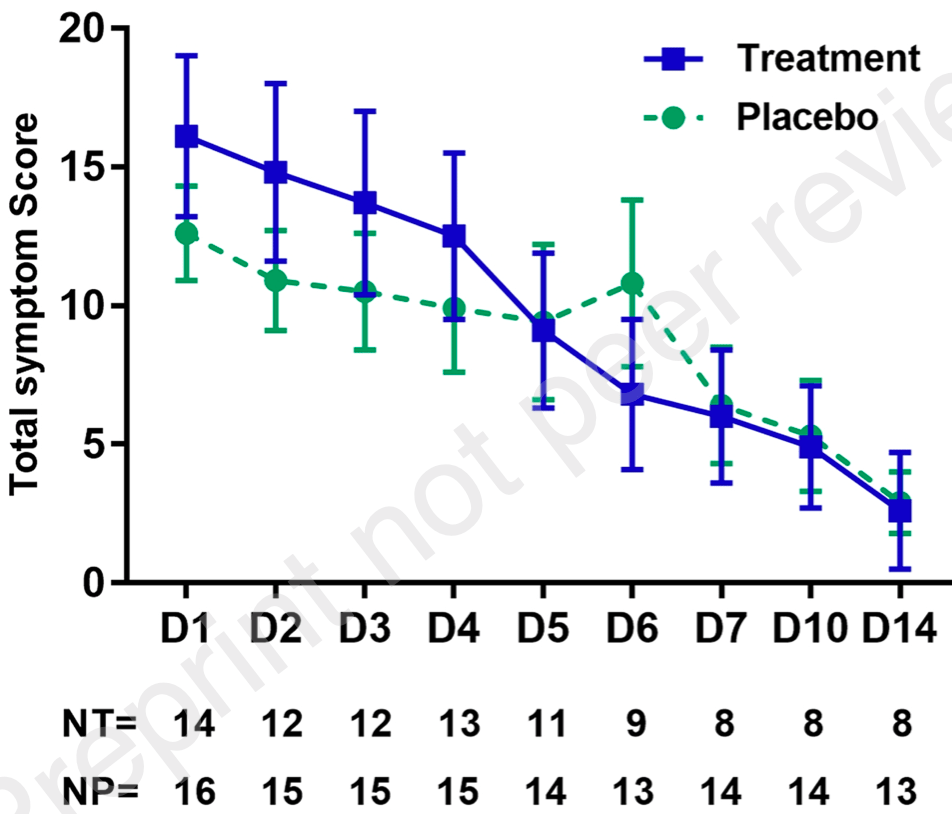
Small RCT of zinc plus resveratrol in COVID-19+ outpatients, showing no significant differences in viral clearance or symptoms. Although the treatment group was older (46.3 vs. 38.5) and had more severe baseline symptoms, they had similar symptomatic recovery by the second week.
The SSRN manuscript ID is "EBIOM-D-21-03006," which is EBioMedicine's submission numbering format, suggesting that the paper may have been rejected by EBioMedicine and published elswhere.
The treatment group had a much wider age range (20-73) compared to the placebo group (18-55), leading to a significant difference in variance (Levene's test p=0.002). While acknowledged by the authors, this baseline asymmetry suggests randomization did not fully balance the groups in this small cohort. Although the authors performed a post-hoc adjustment for age, the imbalance in a very small sample size limits the robustness of the findings.
Co-author JDG reports receiving research support, grants, and personal consulting/speaker fees from multiple major pharmaceutical companies (Lilly, Gilead, Regeneron, Viracor, Mylan).
In Table 1, under the treatment group (N=14), 'not Hispanic/LatinX' is listed as '13 (39%)', where 39% is likely an error for 93%.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers resveratrol and zinc.
|
risk of mechanical ventilation, 14.3% higher, RR 1.14, p = 1.00, treatment 1 of 14 (7.1%), control 1 of 16 (6.2%).
|
|
risk of ICU admission, 14.3% higher, RR 1.14, p = 1.00, treatment 1 of 14 (7.1%), control 1 of 16 (6.2%).
|
|
risk of hospitalization, 14.3% higher, RR 1.14, p = 1.00, treatment 1 of 14 (7.1%), control 1 of 16 (6.2%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kaplan et al., 1 Oct 2021, Randomized Controlled Trial, USA, peer-reviewed, 12 authors, study period 21 September, 2020 - 22 January, 2021, average treatment delay 5.9 days, this trial uses multiple treatments in the treatment arm (combined with resveratrol) - results of individual treatments may vary, trial NCT04542993 (history) (Reszinate).
Zinc and resveratrol treatment of outpatients with COVID-19 -The Reszinate study. A Phase 1/2 randomized clinical trial utilizing home patient-obtained nasal and saliva viral sampling
Background Safe, effective, inexpensive treatment for COVID-19 is an urgent unmet medical need. Zinc and resveratrol have been reported to have antiviral activity and resveratrol may increase zinc activity at the site of replication by increasing intracellular zinc concentrations.
Methods A 1:1 randomized, placebo-controlled trial of zinc 150 mg plus resveratrol 4 grams daily for 5 days versus placebos in SARS-CoV-2 positive outpatients was carried out 9/21/2020 -1/22/2021 in Seattle, Washington. Patients were enrolled within four days of testing positive if they had no chronic liver, kidney, or lung disease and did not have hypoxia requiring supplemental oxygen. Viral shedding was followed at days 1-7,10, and 14 with patient self-collected nasal and saliva samples by measuring qRT-PCR for SARS-CoV-2 N gene. Patients filled out a web-based questionnaire on days 1-14 to report symptoms, vital signs and adherence to study intervention. Findings 45 persons consented to enrollment, and 30 (14 treatment; 16 placebo) had ≥1 day of the protocol treatment and were evaluable for the primary or secondary outcome. There was no difference in viral shedding between groups. There was a nonstatistically significant trend toward more rapid decrease in symptoms in the treatment group. Viral shedding was similar between patient self-collected mid-turbinate nasal swabs and expectorated saliva samples with good correlation, R= 0.67, p<0.001. Interpretation SARS-CoV-2 shedding and COVID-19 symptoms were not statistically significantly decreased by treatment in this small Phase 1/2 pilot study. Viral shedding correlates well between patient-obtained home nasal swab and saliva sampling.
Author contributions: Conceptualization: HGK, CCN, DAK, JMS, JDG. Data Curation: HGK, KW, KMR, JMS, RJ. Analysis: JMS, RJ. Investigation: all authors. Funding: HGK. Methodology: HGK, KW, CCN, JMS, RJ, JDG. Supervision: HGK, JDG. Writing -original draft: HGK. Writing -review and editing: all authors. Final approval of the manuscript: all authors.
Supporting information:
CONSORT checklist Figure 1
References
Altamirano, Govindarajan, Bloomkalns, Assessment of sensitivity and specificity of patient-collected lower nasal specimens for severe acute respiratory syndrome Coronavirus 2 testing, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.12005
Bannerman, Julvez, Oarga, Integrated human/SARS-CoV-2 metabolic models present novel treatment strategies against COVID-19, Life Sci Alliance, doi:10.26508/lsa.202000954
Bastos, Perlman-Arrow, Menzies, The sensitivity and costs of testing for SARS-CoV-2 infection with saliva versus nasopharyngeal swabs, Ann Int Med
Bonaventura, Benedetti, Albarede, Miossec, Zinc and its role in immunity and inflammation, Autoimmun Rev
Butler-Laporte, Lawandi, Schiller, Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-COV-2: A systematic review and meta-analysis, JAMA Int Med, doi:10.1001/jamainternalmed.2020.8876
Carlucci, Ahuja, Petrilli, Rajagopalan, Jones et al., Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients, J Med Microbiol
Core, R: A language and environment for statistical computing
Da Silva, Marinho, Lcn, Silva, Saliva as a possible tool for the SARS-CoV-2 Detection: A review, Travel Med Inf Dis, doi:10.1016/j.tmaid.2020.101920
Fosmire, Zinc toxicity, Amer J Clin Nutr
Gammoh, Rink, Zinc in infection and inflammation, Nutrients, doi:10.3390/nu9060624
Hanson, Barker, Hillyard, Dr, Self-collected anterior nasal and saliva specimens versus health care worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2, J Clin Micro, doi:10.1128/JCM.01824-20
He, Zhu, Yu, Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms, Pharmacol Res, doi:10.1016/jphre.2020.105224
Hoang, An approach of fatty acids and resveratrol in the prevention of COVID-19 severity, Phtother Res, doi:10.1002/ptr.6956
Iwanami, Ejima, Kim, Detection of signficiant antiviral drug effects on COVID-19 with reasonable sample sizes in randomized controlled trials: A modeling study, PLoS Med, doi:10.1371/journal.pmed.1003660
Jothimani, Kailasam, Danielraj, COVID-19: Poor outcomes in patients with zinc deficiency, Intl J Inf Dis
Joung, Ladha, Saito, Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing, N Eng J Med
Kaushik, Subramani, Anang, Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase, J Virol, doi:10.1128/JVI.00754-17
Kivela, Jarva, Lappalalnen, Saliva-based testing for diagnosis of SARS-CoV-2 infection: A meta-analysis, J Med Virol
Krenn, Gaudernak, Holzer, Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections, J Virol
Kvamme, Grønli, Jacobsen, Florholmen, Risk of malnutrition and zinc deficiency in community-living elderly men and women: the Tromsø Study, Pub Health Nutr
Lee, Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19, Nat Biotechnol, doi:10.1038/s41587-021-01020-4
Levy, Delvin, Marcil, Spahis, Can phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (COVID-19), Am J Physiol Endo Metab, doi:.org/10.1152/ajpendo.00298.2020
Lindner, Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab
Malaguernera, Influence of resveratrol on the immune repsonse, Nutrients, doi:10.3390/nu
Mcculloch, Kim, Wilcox, Comparison of unsupervised home selfcollected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.16.382.doi.org/10.1001/jamanetworkopen.2020.16382
Mortaz, Bezemer, Alipoor, Sd, Nutritional impact and its potential consequences on COVID-19 severity, Front Nutri, doi:.org/10.3389/fnut.2021.698617
Nagura-Ikeda, Imal, Tabata, Clinical evaluation of self-collected saliva by quantitative reverse transcription-PCR (RT-qPCR), direct RT-qPCR, reverse transcription-loopmediated isothermal amplification, and a rapid antigen test to diagnose COVID-19, J Clin Micro
Olechnowicz, Tinkov, Skalny, Suliburska, Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism, J Physiol Sci
Ott, Strine, Watkins, Simply saliva: stability of SARS-CoV-2 detection negates the need for expensive collection devices, medRxiv
Pal, Squitti, Picozza, Zinc and COvid-19: Basis of Current Clinical Trials, Biol Trace Elem Res
Read, Obeid, Ahlenstiel, Ahlenstiel, The role of zinc in antiviral immunity, Adv Nutr
Sammans, Roberts, The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers, Med J Austria
Silva, Lucas, Sundaram, Saliva viralload is a dynamic unifying correlate of COVID-19 severity and mortality, MedRxiv, doi:10.1101/2021.01.04.21249236
Suara, Jr, Je, Effect of zinc Salts on respiratory syncytial virus replication, Antimicrob Agents Chemother
Tan, Tey, Lim, The accuracy of health care worker versus self collected (2-in-1) oropharyngeal and bilateral mid-turbinate (OPMT) swabs and saliva samples for SARS-CoV-2, J Infect, doi:10.1371/journal.pone.0244417
Teo, Choudhury, Beehaut, Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 Infection, Sci Rep, doi:10.1038/s41598-021-82787-z
Thomas, Patel, Bittel, Effect of high dose zinc and ascorbic acid supplementation versus usual care on symptom length and reduction In ambulatory patients with Sars-CoV-2 infection. The COVID A to Z randomzied clinical trial, JAMA Open Netw
Uyeki, Bernstein, Hh, Bradley, Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak Management of seasonal influenza A, Clin Infect Dis, doi:10.1093/ci/ciz044.Thispreprintresearchpaperhasnotbeenpeerreviewed
Velthuis, Van Den Worm, Sims, Baric, Snijder et al., None
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combintion and outcomes i outpatients with COVID-19, N Eng J Med, doi:10.1056/NEJMoa2108163
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Wessels, Rolles, Rink, The Potential Impact of zinc supplementation on COVID-19 pathogenesis, Frontiers in Immunology
Wyllie, Fournier, Casanova-Massana, Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, N Eng J Med
Wölfel, Virological assessment of hospitalized patients with COVID
Xue, Moyer, Peng, Wu, Hannafon et al., Chloroquine is a zinc ionophore, PloS One, doi:10.1371/journal.pone.0109180
Zhang, Wu, Schoene, Effect of resveratrol and zinc on intracellular zinc status in normal human prostate epithelial cells, Amer J Physiol Cell Physiol, doi:10.1152/ajpcell.00139.2009
