Ritonavir Has Reproductive Toxicity Depending on Disrupting PI3K/PDK1/AKT Signaling Pathway
et al., Toxics, doi:10.3390/toxics12010073, Jan 2024
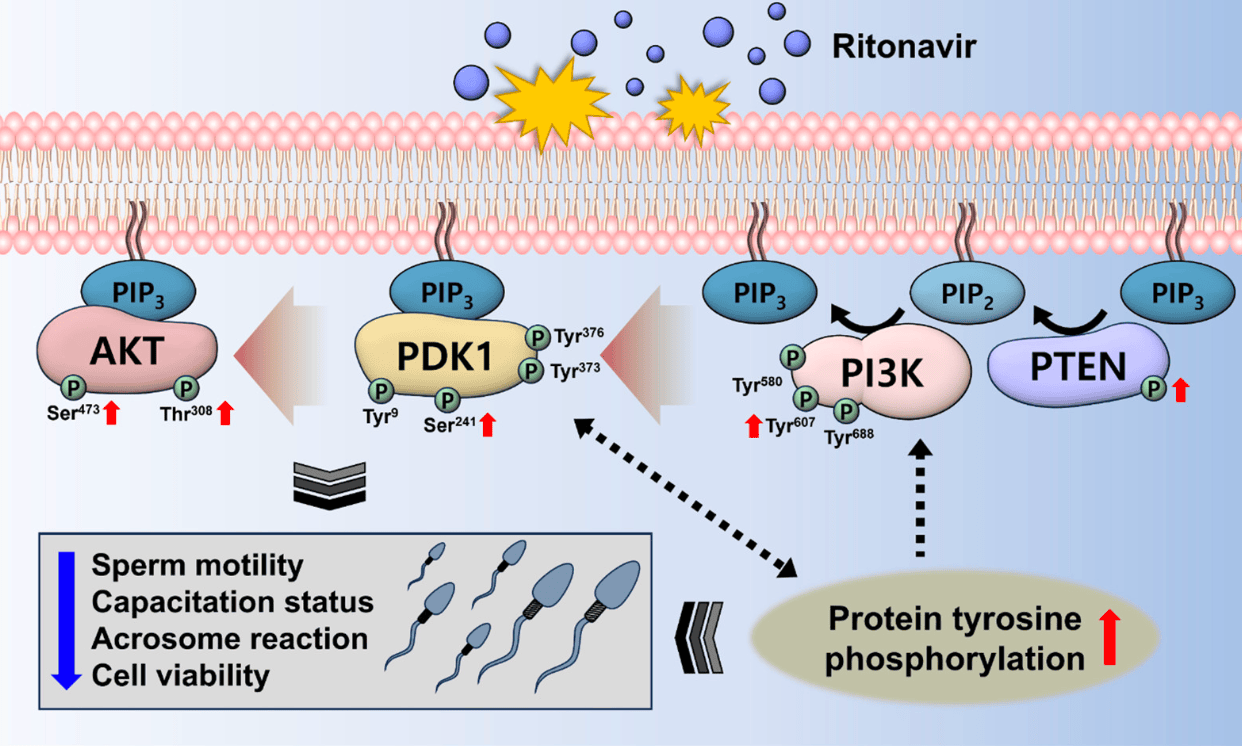
In vitro study on boar spermatozoa showing that the HIV drug ritonavir (part of paxlovid and xiannuoxin) causes reproductive toxicity by disrupting the PI3K/PDK1/AKT signaling pathway. Ritonavir suppressed sperm functions including motility, viability, and capacitation status. The underlying mechanism appears to involve increased abnormal tyrosine phosphorylation which alters PI3K/PDK1/AKT signaling. Specifically, ritonavir increased phosphorylation of PI3K, PDK1, AKT and PTEN while decreasing total PI3K. The authors suggest those using or prescribing ritonavir consider potential male reproductive toxicity.
3 preclinical studies support the efficacy of xiannuoxin for COVID-19:
Study covers paxlovid and xiannuoxin.
Jung et al., 15 Jan 2024, peer-reviewed, 8 authors.
Contact: wskwon@knu.ac.kr (corresponding author), red0787@naver.com, claudineuwa20@gmail.com, todwnl5787@naver.com, wj9059lee@naver.com, ghkdwnal100@gmail.com, jwbae1822@gmail.com, ocjallk@naver.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Ritonavir Has Reproductive Toxicity Depending on Disrupting PI3K/PDK1/AKT Signaling Pathway
Toxics, doi:10.3390/toxics12010073
Ritonavir (RTV) is an antiviral and a component of COVID-19 treatments. Moreover, RTV demonstrates anti-cancer effects by suppressing AKT. However, RTV has cytotoxicity and suppresses sperm functions by altering AKT activity. Although abnormal AKT activity is known for causing detrimental effects on sperm functions, how RTV alters AKT signaling in spermatozoa remains unknown. Therefore, this study aimed to investigate reproductive toxicity of RTV in spermatozoa through phosphoinositide 3-kinase/phosphoinositide-dependent protein kinase-1/protein kinase B (PI3K/PDK1/AKT) signaling. Duroc spermatozoa were treated with various concentrations of RTV, and capacitation was induced. Sperm functions (sperm motility, motion kinematics, capacitation status, and cell viability) and expression levels of tyrosine-phosphorylated proteins and PI3K/PDK1/AKT pathway-related proteins were evaluated. In the results, RTV significantly suppressed sperm motility, motion kinematics, capacitation, acrosome reactions, and cell viability. Additionally, RTV significantly increased levels of phospho-tyrosine proteins and PI3K/PDK1/AKT pathway-related proteins except for AKT and PI3K. The expression level of AKT was not significantly altered and that of PI3K was significantly decreased. These results suggest RTV may suppress sperm functions by induced alterations of PI3K/PDK1/AKT pathway through abnormally increased tyrosine phosphorylation. Therefore, we suggest people who use or prescribe RTV need to consider its male reproductive toxicity.
Toxics 2024, 12, 73 9 of 11 aberrant tyrosine phosphorylation may increase the phosphorylation of PI3K and PDK1, which may enhance AKT phosphorylation (Figure 4 ). Taken together, RTV is predicted to have reproductive toxicity by disrupting PI3K/PDK1/AKT signaling pathway. Therefore, we suggest when using or administering RTV, particular attention be given to male reproductive toxicity. The findings of this study are anticipated to support the further investigation of PI3K/PDK1/AKT signaling.
Supplementary Materials: The following supporting information can be downloaded at: https://www. mdpi.com/article/10.3390/toxics12010073/s1, Figure S1
References
Alessi, Andjelkovic, Caudwell, Cron, Morrice et al., Mechanism of activation of protein kinase B by insulin and IGF-1, EMBO J, doi:10.1002/j.1460-2075.1996.tb01045.x
Alessi, James, Downes, Holmes, Gaffney et al., Characterization of a 3-phosphoinositidedependent protein kinase which phosphorylates and activates protein kinase Bα, Curr. Biol, doi:10.1016/S0960-9822(06)00122-9
Aparicio, Bragado, Gil, Garcia-Herreros, Gonzalez-Fernandez et al., Phosphatidylinositol 3-kinase pathway regulates sperm viability but not capacitation on boar spermatozoa, Mol. Reprod. Dev, doi:10.1002/mrd.20663
Arend, Brandmann, Dringen, The antiretroviral protease inhibitor ritonavir accelerates glutathione export from cultured primary astrocytes, Neurochem. Res, doi:10.1007/s11064-013-0971-x
Austin, Observations on the penetration of the sperm into the mammalian egg, Aust. J. Biol. Sci, doi:10.1071/BI9510581
Austin, The 'capacitation' of the mammalian sperm, Nature, doi:10.1038/170326a0
Baden, Rubin, COVID-19-The search for effective therapy, N. Engl. J. Med, doi:10.1056/NEJMe2005477
Bae, Kwon, Investigating the effects of fipronil on male fertility: Insight into the mechanism of capacitation, Reprod. Toxicol, doi:10.1016/j.reprotox.2020.04.002
Batchu, Gruzdyn, Bryant, Qazi, Kumar et al., Ritonavir-Mediated Induction of Apoptosis in Pancreatic Cancer Occurs via the RB/E2F-1 and AKT Pathways, Pharmaceuticals, doi:10.3390/ph7010046
Breitbart, Rotman, Rubinstein, Etkovitz, Role and regulation of PI3K in sperm capacitation and the acrosome reaction, Mol. Cell. Endocrinol, doi:10.1016/j.mce.2009.06.009
Chang, Fertilizing capacity of spermatozoa deposited into the fallopian tubes, Nature, doi:10.1038/168697b0
Dalva-Aydemir, Bajpai, Martinez, Adekola, Kandela et al., Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin, Clin. Cancer Res, doi:10.1158/1078-0432.CCR-14-1088
Dorostghoal, Galehdari, Moramezi, Danyari, Sperm miR-26a-5p and its target PTEN transcripts content in men with unexplained infertility, Andrology, doi:10.1111/andr.12801
Fisher, Brewis, Barratt, Cooke, Moore, Phosphoinositide 3-kinase is involved in the induction of the human sperm acrosome reaction downstream of tyrosine phosphorylation, Mol. Hum. Reprod, doi:10.1093/molehr/4.9.849
Gallardo Bolanos, Balao Da Silva, Martin Munoz, Morillo Rodriguez, Plaza Davila et al., Phosphorylated AKT preserves stallion sperm viability and motility by inhibiting caspases 3 and 7, Reproduction, doi:10.1530/REP-13-0191
Ganta, Chaubey, Endoplasmic reticulum stress leads to mitochondria-mediated apoptosis in cells treated with anti-HIV protease inhibitor ritonavir, Cell Biol. Toxicol, doi:10.1007/s10565-018-09451-7
Grillo, Grémeaux, Casamayor, Alessi, Marchand-Brustel et al., Peroxovanadate induces tyrosine phosphorylation of phosphoinositide-dependent protein kinase-1: Potential involvement of Src kinase, Eur. J. Biochem, doi:10.1046/j.1432-1327.2000.01759.x
Harayama, Nakamura, Changes of PKA and PDK1 in the principal piece of boar spermatozoa treated with a cell-permeable cAMP analog to induce flagellar hyperactivation, Mol. Reprod. Dev, doi:10.1002/mrd.20882
Hwang, Bae, Jung, Lee, Kwon et al., Has Detrimental Effects on Sperm Functions, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph19010061
Jang, Jo, Jung, Lee, Hwang et al., Development of an optimal protocol to induce capacitation of boar spermatozoa in vitro, J. Anim. Reprod. Biotechnol, doi:10.12750/JARB.37.4.285
Jung, Lee, Hwang, Bae, Kwon, Reproductive Toxicity of Ritonavir in Male: Insight into mouse sperm capacitation, Reprod. Toxicol, doi:10.1016/j.reprotox.2022.09.008
Kempf, Marsh, Denissen, Mcdonald, Vasavanonda et al., ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans, Proc. Natl. Acad. Sci, doi:10.1073/pnas.92.7.2484
Kim, Bae, Kim, Jeong, Ha et al., Detrimental effects of temephos on male fertility: An in vitro study on a mouse model, Reprod. Toxicol, doi:10.1016/j.reprotox.2020.06.008
Koppers, Mitchell, Wang, Lin, Aitken, Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa, Biochem. J, doi:10.1042/BJ20110114
Kumar, Bryant, Chamala, Qazi, Seward et al., Ritonavir blocks AKT signaling, activates apoptosis and inhibits migration and invasion in ovarian cancer cells, Mol. Cancer, doi:10.1186/1476-4598-8-26
Lan, Kiyota, Hanamsagar, Huang, Andrews et al., The effect of HIV protease inhibitors on amyloid-β peptide degradation and synthesis in human cells and Alzheimer's disease animal model, J. Neuroimmune Pharmacol, doi:10.1007/s11481-011-9304-5
Lee, Jo, Jang, Jung, Hwang et al., The Natural Flavonoid Compound Deguelin Suppresses Sperm (Sus Scrofa) Functions through Abnormal Activation of the PI3K/AKT Pathway, Reprod. Toxicol, doi:10.1016/j.reprotox.2023.108426
Lee, Jung, Hwang, Bae, Kwon, GRP78 plays a key role in sperm function via the PI3K/PDK1/AKT pathway, Reprod. Toxicol, doi:10.1016/j.reprotox.2022.08.008
Li, Chai, Chen, Zhang, Tan-Tai et al., Prohibitin (PHB) interacts with AKT in mitochondria to coordinately modulate sperm motility, Asian J. Androl, doi:10.4103/aja.aja_46_20
Ma, Fan, Zhang, Feng, Hu et al., Testosterone-dependent miR-26a-5p and let-7g-5p act as signaling mediators to regulate sperm apoptosis via targeting PTEN and PMAIP1, Int. J. Mol. Sci, doi:10.3390/ijms19041233
Macías-García, García-Marín, Bragado, González-Fernández, The calcium-sensing receptor regulates protein tyrosine phosphorylation through PDK1 in boar spermatozoa, Mol. Reprod. Dev, doi:10.1002/mrd.23160
Mahase, COVID-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ, doi:10.1136/bmj.n2713
Martinez, Compounds with therapeutic potential against novel respiratory 2019 coronavirus, Antimicrob. Agents Chemother, doi:10.1128/AAC.00399-20
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Recommendations for the management of drug-drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications, Clin. Pharmacol. Ther, doi:10.1002/cpt.2646
Park, Hill, Hess, Brazil, Hofsteenge et al., Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity, J. Biol. Chem, doi:10.1074/jbc.M105916200
Quan, Liu, Effect of Akti-2 on sperm motility, capacitation and acrosome reaction in a mouse model, Biomed. Rep, doi:10.3892/br.2016.627
Srirangam, Mitra, Wang, Gorski, Badve et al., Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer, Clin. Cancer Res, doi:10.1158/1078-0432.CCR-05-1167
Stambolic, Suzuki, De La Pompa, Brothers, Mirtsos et al., Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN, Cell, doi:10.1016/S0092-8674(00)81780-8
Von Willebrand, Williams, Saxena, Gilman, Tailor et al., Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl-or Lck-mediated tyrosine phosphorylation at Tyr-688, J. Biol. Chem, doi:10.1074/jbc.273.7.3994
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Wei, Faccio, Takeshita, Tebas et al., The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling, J. Clin. Investig, doi:10.1172/JCI15797
Yang, Shin, Piao, Shin, Li et al., Regulation of 3phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding, J. Biol. Chem, doi:10.1074/jbc.M706361200
Zhang, Wu, Zhao, Xu, Zhang et al., Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways, Toxicol. Appl. Pharmacol, doi:10.1016/j.taap.2017.12.011
Zhao, Zhang, Liu, Zhang, Hao et al., Hydrogen sulfide and/or ammonia reduces spermatozoa motility through AMPK/AKT related pathways, Sci. Rep, doi:10.1038/srep37884
Zhong, Lu, Conklin, Lin, Lumsden et al., HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells, Arterioscler. Thromb. Vasc. Biol, doi:10.1161/01.ATV.0000034707.40046.02
DOI record:
{
"DOI": "10.3390/toxics12010073",
"ISSN": [
"2305-6304"
],
"URL": "http://dx.doi.org/10.3390/toxics12010073",
"abstract": "<jats:p>Ritonavir (RTV) is an antiviral and a component of COVID-19 treatments. Moreover, RTV demonstrates anti-cancer effects by suppressing AKT. However, RTV has cytotoxicity and suppresses sperm functions by altering AKT activity. Although abnormal AKT activity is known for causing detrimental effects on sperm functions, how RTV alters AKT signaling in spermatozoa remains unknown. Therefore, this study aimed to investigate reproductive toxicity of RTV in spermatozoa through phosphoinositide 3-kinase/phosphoinositide-dependent protein kinase-1/protein kinase B (PI3K/PDK1/AKT) signaling. Duroc spermatozoa were treated with various concentrations of RTV, and capacitation was induced. Sperm functions (sperm motility, motion kinematics, capacitation status, and cell viability) and expression levels of tyrosine-phosphorylated proteins and PI3K/PDK1/AKT pathway-related proteins were evaluated. In the results, RTV significantly suppressed sperm motility, motion kinematics, capacitation, acrosome reactions, and cell viability. Additionally, RTV significantly increased levels of phospho-tyrosine proteins and PI3K/PDK1/AKT pathway-related proteins except for AKT and PI3K. The expression level of AKT was not significantly altered and that of PI3K was significantly decreased. These results suggest RTV may suppress sperm functions by induced alterations of PI3K/PDK1/AKT pathway through abnormally increased tyrosine phosphorylation. Therefore, we suggest people who use or prescribe RTV need to consider its male reproductive toxicity.</jats:p>",
"alternative-id": [
"toxics12010073"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7122-2004",
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Jung",
"given": "Eun-Ju",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Animal Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"family": "Jo",
"given": "Jae-Hwan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"family": "Uwamahoro",
"given": "Claudine",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0188-8297",
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Jang",
"given": "Seung-Ik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"family": "Lee",
"given": "Woo-Jin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1776-9759",
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Hwang",
"given": "Ju-Mi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7030-7767",
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Bae",
"given": "Jeong-Won",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0848-7189",
"affiliation": [
{
"name": "Department of Animal Science and Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
},
{
"name": "Department of Animal Biotechnology, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
},
{
"name": "Research Institute for Innovative Animal Science, Kyungpook National University, Sangju 37224, Gyeongsangbuk-do, Republic of Korea"
}
],
"authenticated-orcid": false,
"family": "Kwon",
"given": "Woo-Sung",
"sequence": "additional"
}
],
"container-title": "Toxics",
"container-title-short": "Toxics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T16:15:01Z",
"timestamp": 1705335301000
},
"deposited": {
"date-parts": [
[
2024,
1,
16
]
],
"date-time": "2024-01-16T11:15:15Z",
"timestamp": 1705403715000
},
"indexed": {
"date-parts": [
[
2024,
1,
17
]
],
"date-time": "2024-01-17T00:37:49Z",
"timestamp": 1705451869870
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T00:00:00Z",
"timestamp": 1705276800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2305-6304/12/1/73/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "73",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
1,
15
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
15
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1073/pnas.92.7.2484",
"article-title": "ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans",
"author": "Kempf",
"doi-asserted-by": "crossref",
"first-page": "2484",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_1",
"volume": "92",
"year": "1995"
},
{
"DOI": "10.1128/AAC.00399-20",
"article-title": "Compounds with therapeutic potential against novel respiratory 2019 coronavirus",
"author": "Martinez",
"doi-asserted-by": "crossref",
"first-page": "e00399-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_2",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1056/NEJMe2005477",
"article-title": "COVID-19—The search for effective therapy",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "1851",
"journal-title": "N. Engl. J. Med.",
"key": "ref_3",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "ref_4",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1136/bmj.n2713",
"article-title": "COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2713",
"journal-title": "BMJ",
"key": "ref_5",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1158/1078-0432.CCR-05-1167",
"article-title": "Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer",
"author": "Srirangam",
"doi-asserted-by": "crossref",
"first-page": "1883",
"journal-title": "Clin. Cancer Res.",
"key": "ref_6",
"volume": "12",
"year": "2006"
},
{
"DOI": "10.3390/ph7010046",
"article-title": "Ritonavir-Mediated Induction of Apoptosis in Pancreatic Cancer Occurs via the RB/E2F-1 and AKT Pathways",
"author": "Batchu",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Pharmaceuticals",
"key": "ref_7",
"volume": "7",
"year": "2014"
},
{
"DOI": "10.1161/01.ATV.0000034707.40046.02",
"article-title": "HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells",
"author": "Zhong",
"doi-asserted-by": "crossref",
"first-page": "1560",
"journal-title": "Arterioscler. Thromb. Vasc. Biol.",
"key": "ref_8",
"volume": "22",
"year": "2002"
},
{
"DOI": "10.1007/s10565-018-09451-7",
"article-title": "Endoplasmic reticulum stress leads to mitochondria-mediated apoptosis in cells treated with anti-HIV protease inhibitor ritonavir",
"author": "Ganta",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Cell Biol. Toxicol.",
"key": "ref_9",
"volume": "35",
"year": "2019"
},
{
"DOI": "10.1016/j.reprotox.2022.09.008",
"article-title": "Reproductive Toxicity of Ritonavir in Male: Insight into mouse sperm capacitation",
"author": "Jung",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Reprod. Toxicol.",
"key": "ref_10",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1038/170326a0",
"article-title": "The ‘capacitation’ of the mammalian sperm",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "326",
"journal-title": "Nature",
"key": "ref_11",
"volume": "170",
"year": "1952"
},
{
"DOI": "10.1071/BI9510581",
"article-title": "Observations on the penetration of the sperm into the mammalian egg",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "581",
"journal-title": "Aust. J. Biol. Sci.",
"key": "ref_12",
"volume": "4",
"year": "1951"
},
{
"DOI": "10.1038/168697b0",
"article-title": "Fertilizing capacity of spermatozoa deposited into the fallopian tubes",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "697",
"journal-title": "Nature",
"key": "ref_13",
"volume": "168",
"year": "1951"
},
{
"DOI": "10.1016/j.mce.2009.06.009",
"article-title": "Role and regulation of PI3K in sperm capacitation and the acrosome reaction",
"author": "Breitbart",
"doi-asserted-by": "crossref",
"first-page": "234",
"journal-title": "Mol. Cell. Endocrinol.",
"key": "ref_14",
"volume": "314",
"year": "2010"
},
{
"DOI": "10.1093/molehr/4.9.849",
"article-title": "Phosphoinositide 3-kinase is involved in the induction of the human sperm acrosome reaction downstream of tyrosine phosphorylation",
"author": "Fisher",
"doi-asserted-by": "crossref",
"first-page": "849",
"journal-title": "Mol. Hum. Reprod.",
"key": "ref_15",
"volume": "4",
"year": "1998"
},
{
"DOI": "10.1042/BJ20110114",
"article-title": "Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa",
"author": "Koppers",
"doi-asserted-by": "crossref",
"first-page": "687",
"journal-title": "Biochem. J.",
"key": "ref_16",
"volume": "436",
"year": "2011"
},
{
"DOI": "10.12750/JARB.37.4.285",
"article-title": "Development of an optimal protocol to induce capacitation of boar spermatozoa in vitro",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "285",
"journal-title": "J. Anim. Reprod. Biotechnol.",
"key": "ref_17",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1016/j.reprotox.2022.08.008",
"article-title": "GRP78 plays a key role in sperm function via the PI3K/PDK1/AKT pathway",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "103",
"journal-title": "Reprod. Toxicol.",
"key": "ref_18",
"volume": "113",
"year": "2022"
},
{
"DOI": "10.1016/j.reprotox.2023.108426",
"article-title": "The Natural Flavonoid Compound Deguelin Suppresses Sperm (Sus Scrofa) Functions through Abnormal Activation of the PI3K/AKT Pathway",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "108426",
"journal-title": "Reprod. Toxicol.",
"key": "ref_19",
"volume": "120",
"year": "2023"
},
{
"DOI": "10.1007/s11064-013-0971-x",
"article-title": "The antiretroviral protease inhibitor ritonavir accelerates glutathione export from cultured primary astrocytes",
"author": "Arend",
"doi-asserted-by": "crossref",
"first-page": "732",
"journal-title": "Neurochem. Res.",
"key": "ref_20",
"volume": "38",
"year": "2013"
},
{
"DOI": "10.1007/s11481-011-9304-5",
"article-title": "The effect of HIV protease inhibitors on amyloid-β peptide degradation and synthesis in human cells and Alzheimer’s disease animal model",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "412",
"journal-title": "J. Neuroimmune Pharmacol.",
"key": "ref_21",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.3390/ijerph19010061",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Hwang, J.-M., Bae, J.-W., Jung, E.-J., Lee, W.-J., and Kwon, W.-S. (2021). Novaluron Has Detrimental Effects on Sperm Functions. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.1016/j.reprotox.2020.06.008",
"article-title": "Detrimental effects of temephos on male fertility: An in vitro study on a mouse model",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "150",
"journal-title": "Reprod. Toxicol.",
"key": "ref_23",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1016/j.reprotox.2020.04.002",
"article-title": "Investigating the effects of fipronil on male fertility: Insight into the mechanism of capacitation",
"author": "Bae",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Reprod. Toxicol.",
"key": "ref_24",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1002/cpt.2646",
"article-title": "Recommendations for the management of drug–drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "1191",
"journal-title": "Clin. Pharmacol. Ther.",
"key": "ref_25",
"volume": "112",
"year": "2022"
},
{
"DOI": "10.1172/JCI15797",
"article-title": "The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "206",
"journal-title": "J. Clin. Investig.",
"key": "ref_26",
"volume": "114",
"year": "2004"
},
{
"DOI": "10.1158/1078-0432.CCR-14-1088",
"article-title": "Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin",
"author": "Bajpai",
"doi-asserted-by": "crossref",
"first-page": "1161",
"journal-title": "Clin. Cancer Res.",
"key": "ref_27",
"volume": "21",
"year": "2015"
},
{
"DOI": "10.1186/1476-4598-8-26",
"article-title": "Ritonavir blocks AKT signaling, activates apoptosis and inhibits migration and invasion in ovarian cancer cells",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Mol. Cancer",
"key": "ref_28",
"volume": "8",
"year": "2009"
},
{
"DOI": "10.1016/S0092-8674(00)81780-8",
"article-title": "Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN",
"author": "Stambolic",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Cell",
"key": "ref_29",
"volume": "95",
"year": "1998"
},
{
"DOI": "10.1016/S0960-9822(06)00122-9",
"article-title": "Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα",
"author": "Alessi",
"doi-asserted-by": "crossref",
"first-page": "261",
"journal-title": "Curr. Biol.",
"key": "ref_30",
"volume": "7",
"year": "1997"
},
{
"DOI": "10.1002/j.1460-2075.1996.tb01045.x",
"article-title": "Mechanism of activation of protein kinase B by insulin and IGF-1",
"author": "Alessi",
"doi-asserted-by": "crossref",
"first-page": "6541",
"journal-title": "EMBO J.",
"key": "ref_31",
"volume": "15",
"year": "1996"
},
{
"DOI": "10.1038/srep37884",
"article-title": "Hydrogen sulfide and/or ammonia reduces spermatozoa motility through AMPK/AKT related pathways",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "37884",
"journal-title": "Sci. Rep.",
"key": "ref_32",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1111/andr.12801",
"article-title": "Sperm miR-26a-5p and its target PTEN transcripts content in men with unexplained infertility",
"author": "Dorostghoal",
"doi-asserted-by": "crossref",
"first-page": "1167",
"journal-title": "Andrology",
"key": "ref_33",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/ijms19041233",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Ma, J., Fan, Y., Zhang, J., Feng, S., Hu, Z., Qiu, W., Long, K., Jin, L., Tang, Q., and Wang, X. (2018). Testosterone-dependent miR-26a-5p and let-7g-5p act as signaling mediators to regulate sperm apoptosis via targeting PTEN and PMAIP1. Int. J. Mol. Sci., 19."
},
{
"DOI": "10.1002/mrd.20663",
"article-title": "Phosphatidylinositol 3-kinase pathway regulates sperm viability but not capacitation on boar spermatozoa",
"author": "Aparicio",
"doi-asserted-by": "crossref",
"first-page": "1035",
"journal-title": "Mol. Reprod. Dev.",
"key": "ref_35",
"volume": "74",
"year": "2007"
},
{
"DOI": "10.4103/aja.aja_46_20",
"article-title": "Prohibitin (PHB) interacts with AKT in mitochondria to coordinately modulate sperm motility",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "583",
"journal-title": "Asian J. Androl.",
"key": "ref_36",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1016/j.taap.2017.12.011",
"article-title": "Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "Toxicol. Appl. Pharmacol.",
"key": "ref_37",
"volume": "340",
"year": "2018"
},
{
"DOI": "10.1530/REP-13-0191",
"article-title": "Phosphorylated AKT preserves stallion sperm viability and motility by inhibiting caspases 3 and 7",
"author": "Aparicio",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Reproduction",
"key": "ref_38",
"volume": "148",
"year": "2014"
},
{
"DOI": "10.3892/br.2016.627",
"article-title": "Effect of Akti-2 on sperm motility, capacitation and acrosome reaction in a mouse model",
"author": "Quan",
"doi-asserted-by": "crossref",
"first-page": "578",
"journal-title": "Biomed. Rep.",
"key": "ref_39",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.1002/mrd.23160",
"article-title": "The calcium-sensing receptor regulates protein tyrosine phosphorylation through PDK1 in boar spermatozoa",
"author": "Bragado",
"doi-asserted-by": "crossref",
"first-page": "751",
"journal-title": "Mol. Reprod. Dev.",
"key": "ref_40",
"volume": "86",
"year": "2019"
},
{
"DOI": "10.1002/mrd.20882",
"article-title": "Changes of PKA and PDK1 in the principal piece of boar spermatozoa treated with a cell-permeable cAMP analog to induce flagellar hyperactivation",
"author": "Harayama",
"doi-asserted-by": "crossref",
"first-page": "1396",
"journal-title": "Mol. Reprod. Dev.",
"key": "ref_41",
"volume": "75",
"year": "2008"
},
{
"DOI": "10.1074/jbc.273.7.3994",
"article-title": "Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl-or Lck-mediated tyrosine phosphorylation at Tyr-688",
"author": "Williams",
"doi-asserted-by": "crossref",
"first-page": "3994",
"journal-title": "J. Biol. Chem.",
"key": "ref_42",
"volume": "273",
"year": "1998"
},
{
"DOI": "10.1074/jbc.M105916200",
"article-title": "Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "37459",
"journal-title": "J. Biol. Chem.",
"key": "ref_43",
"volume": "276",
"year": "2001"
},
{
"DOI": "10.1046/j.1432-1327.2000.01759.x",
"article-title": "Peroxovanadate induces tyrosine phosphorylation of phosphoinositide-dependent protein kinase-1: Potential involvement of Src kinase",
"author": "Grillo",
"doi-asserted-by": "crossref",
"first-page": "6642",
"journal-title": "Eur. J. Biochem.",
"key": "ref_44",
"volume": "267",
"year": "2000"
},
{
"DOI": "10.1074/jbc.M706361200",
"article-title": "Regulation of 3-phosphoinositide-dependent protein kinase-1 (PDK1) by Src involves tyrosine phosphorylation of PDK1 and Src homology 2 domain binding",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "1480",
"journal-title": "J. Biol. Chem.",
"key": "ref_45",
"volume": "283",
"year": "2008"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2305-6304/12/1/73"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Chemical Health and Safety",
"Health, Toxicology and Mutagenesis",
"Toxicology"
],
"subtitle": [],
"title": "Ritonavir Has Reproductive Toxicity Depending on Disrupting PI3K/PDK1/AKT Signaling Pathway",
"type": "journal-article",
"volume": "12"
}
jung2