HIV Protease Inhibitor Ritonavir Induces Cytotoxicity of Human Endothelial Cells
et al., Arteriosclerosis, Thrombosis, and Vascular Biology, doi:10.1161/01.atv.0000034707.40046.02, Aug 2002
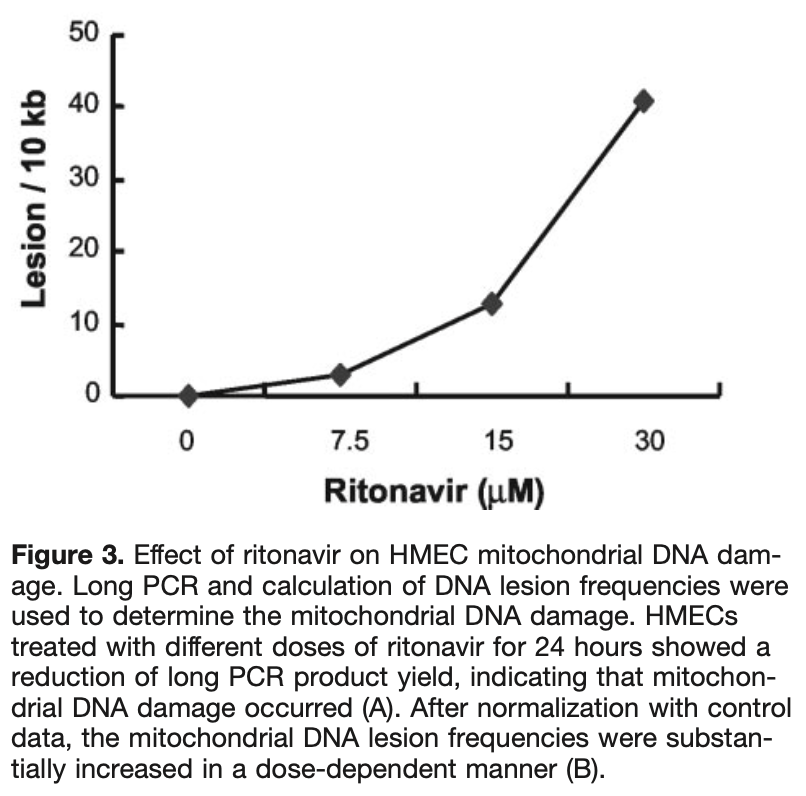
In vitro study showing that ritonavir (part of paxlovid) can cause endothelial mitochondrial DNA damage and cell death at concentrations near clinical plasma levels.
Zhong et al., 22 Aug 2002, peer-reviewed, 7 authors.
Contact: jchen@bcm.tmc.edu.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
HIV Protease Inhibitor Ritonavir Induces Cytotoxicity of Human Endothelial Cells
Arteriosclerosis, Thrombosis, and Vascular Biology, doi:10.1161/01.atv.0000034707.40046.02
Objective-Although HIV protease inhibitors have been successfully used against HIV infection, many metabolic side effects and premature cardiovascular diseases are often associated with this therapy. The mechanisms of these complications are not clear. In this study, we investigated the effect of the HIV protease inhibitor ritonavir on human endothelial cell cultures. Methods and Results-By using nonradioactive cell proliferation and cytotoxicity assays, human endothelial cells treated with ritonavir showed a significant decrease in cell viability and an increase in cytotoxicity in a time-and dose-dependent fashion. Mitochondrial DNA was also substantially damaged with ritonavir treatment by long polymerase chain reaction analysis. In contrast, ritonavir had a very limited effect on endothelial apoptosis, as assessed by analyses of DNA fragmentation and cellular caspase-3 activity. Conclusions-These data demonstrate, for the first time, that the HIV protease inhibitor ritonavir at concentrations near clinical plasma levels is able to directly cause endothelial mitochondrial DNA damage and cell death mainly through necrosis pathways but not through apoptosis. This study suggests that HIV protease inhibitor-mediated endothelial injury may contribute to its cardiovascular complications.
References
Ades, Candal, Swerlick, George, Summers et al., HMEC-1: establishment of an immortalized human microvascular endothelial cell line, J Invest Dermatol
Assmann, Schulte, Von Eckardstein, Hypertriglyceridemia and elevated lipoprotein(a) are risk factors for major coronary events in middle-aged men, Am J Cardiol
Ballinger, Patterson, Yan, Doan, Burow et al., Hydrogen peroxide-and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells, Circ Res
Behrens, Schmidt, Meyer, Stoll, Schmidt, Vascular complications associated with the use of HIV protease inhibitors, Lancet
Buchman, Abello, Smith, Bulkley, Induction of heat shock response leads to apoptosis in endothelial cells previously exposed to endotoxin, Am J Physiol
Carr, Cooper, Images in clinical medicine: lipodystrophy associated with an HIV-protease inhibitor, N Engl J Med
Carr, Samaras, Chisholm, Hypothesis: pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidemia, and insulin resistance, Lancet
Chen, Li, Ren, Chen, Ma et al., HIV protease inhibitor ritonavir causes endothelial dysfunction in monkey arteries
Deeks, Smith, Holodniy, Kahn, HIV-1 protease inhibitors: a review for clinicians, JAMA
Driggers, Ledoux, Wilson, Repair of oxidative damage within the mitochondrial DNA of RINr 38 cells, J Biol Chem
Flexner, HIV-protease inhibitors, N Engl J Med
Gallet, Pulik, Genet, Chedin, Hiltgen, Vascular complications associated with the use of HIV protease inhibitors, Lancet
Glleece, Nelson, Does antiretroviral-induced hyperlipidaemia constitute a cardiovascular risk?, J HIV Ther
Golstein, Oicius, Young, Cell death mechanisms and the immune system, Immunol Rev
Griffoths, Pubrez, Morgan, Jones, Whitehouse et al., Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis, J cell Biol
Gulick, Mellors, Havlir, Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy, N Engl J Med
Hadigan, Meigs, Corcoran, Rietschel, Piecuch et al., Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy, Clin Infect Dis
Hammer, Squires, Hughes, A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less, N Engl J Med
Henry, Melroe, Huebsch, Hermundson, Levine et al., Severe premature coronary artery disease with protease inhibitors, Lancet
Ho, Neumann, Perelson, Chen, Leonard et al., Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection, Nature
Hsu, Granneman, Bertz, Ritonavir: clinical pharmacokinetics and interactions with other anti-HIV agents, Clin Pharmacokinet
Kugiyama, Doi, Motoyama, Soejima, Misumi et al., Association of remnant lipoprotein levels with impairment of endothelium-dependent vasomotor function in human coronary arteries, Circulation
Lundman, Eriksson, Schenck-Gustafsson, Karpe, Tornvall, Transient triglyceridemia decreases vascular reactivity in young, healthy men without risk factors for coronary heart disease, Circulation
Mulligan, Grunfeld, Tai, Algren, Pang et al., Hyperlipidemia and insulin resistance are induced by protease inhibitors independent of changes in body composition in patients with HIV infection, J Acquir Immune Defic Syndr
Neunteufl, Heher, Katzenschlager, Wolfl, Kostner et al., Late prognostic value of flow-mediated vasodilation in the brachial artery of patients with chest pain, Am J Cardiol
Periard, Telenti, Sudre, Cheseaux, Halfon et al., Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors: the Swiss HIV Cohort Study, Circulation
Schachinger, Briton, Zeiher, Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease, Circulation
Stein, Klein, Bellehumeur, Mcbride, Wiebe et al., Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction, Circulation
Suwaidi, Hamasaki, Higano, Nishimura, Holmes Dr Jr et al., Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction, Circulation
Takase, Uehata, Akima, Nagai, Nishioka et al., Endothelium-dependent flowmediated vasodilation in coronary and brachial arteries in suspected coronary artery disease, Am J Cardiol
Vittecoq, Escaut, Monsuez, Vascular complications associated with the use of HIV protease inhibitors, Lancet
Wang, Redmond, Watson, Bouchier-Hayes, Cellular mechanisms of endothelial cell death during the systemic inflammatory response syndrome, Surg Forum
Wei, Ghosh, Taylor, Viral dynamics in human immunodeficiency virus type 1 infection, Nature
Yakes, Houten, Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress, Proc Natl Acad Sci U S A
DOI record:
{
"DOI": "10.1161/01.atv.0000034707.40046.02",
"ISSN": [
"1079-5642",
"1524-4636"
],
"URL": "http://dx.doi.org/10.1161/01.atv.0000034707.40046.02",
"abstract": "<jats:p>\n <jats:bold>\n <jats:italic>Objective—</jats:italic>\n </jats:bold>\n Although HIV protease inhibitors have been successfully used against HIV infection, many metabolic side effects and premature cardiovascular diseases are often associated with this therapy. The mechanisms of these complications are not clear. In this study, we investigated the effect of the HIV protease inhibitor ritonavir on human endothelial cell cultures.\n </jats:p>\n <jats:p>\n <jats:bold>\n <jats:italic>Methods and Results—</jats:italic>\n </jats:bold>\n By using nonradioactive cell proliferation and cytotoxicity assays, human endothelial cells treated with ritonavir showed a significant decrease in cell viability and an increase in cytotoxicity in a time- and dose-dependent fashion. Mitochondrial DNA was also substantially damaged with ritonavir treatment by long polymerase chain reaction analysis. In contrast, ritonavir had a very limited effect on endothelial apoptosis, as assessed by analyses of DNA fragmentation and cellular caspase-3 activity.\n </jats:p>\n <jats:p>\n <jats:bold>\n <jats:italic>Conclusions—</jats:italic>\n </jats:bold>\n These data demonstrate, for the first time, that the HIV protease inhibitor ritonavir at concentrations near clinical plasma levels is able to directly cause endothelial mitochondrial DNA damage and cell death mainly through necrosis pathways but not through apoptosis. This study suggests that HIV protease inhibitor–mediated endothelial injury may contribute to its cardiovascular complications.\n </jats:p>",
"alternative-id": [
"10.1161/01.ATV.0000034707.40046.02"
],
"author": [
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Zhong",
"given": "Dian-sheng",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Lu",
"given": "Xiang-huai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Conklin",
"given": "Brian S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Lin",
"given": "Peter H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Lumsden",
"given": "Alan B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Yao",
"given": "Qizhi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Winship Cancer Institute (D.Z.) and Department of Medicine (X.L.), Emory University, Atlanta, Ga, and the Michael E. DeBakey Department of Surgery (B.S.C., P.H.L., A.B.L., Q.Y., C.C.), Baylor College of Medicine, Houston, Tex."
}
],
"family": "Chen",
"given": "Changyi",
"sequence": "additional"
}
],
"container-title": "Arteriosclerosis, Thrombosis, and Vascular Biology",
"container-title-short": "ATVB",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2002,
10,
12
]
],
"date-time": "2002-10-12T01:18:08Z",
"timestamp": 1034385488000
},
"deposited": {
"date-parts": [
[
2022,
3,
17
]
],
"date-time": "2022-03-17T17:15:31Z",
"timestamp": 1647537331000
},
"indexed": {
"date-parts": [
[
2022,
11,
17
]
],
"date-time": "2022-11-17T17:25:52Z",
"timestamp": 1668705952205
},
"is-referenced-by-count": 96,
"issue": "10",
"issued": {
"date-parts": [
[
2002,
10
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2002,
10
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.ahajournals.org/doi/full/10.1161/01.ATV.0000034707.40046.02",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "1560-1566",
"prefix": "10.1161",
"published": {
"date-parts": [
[
2002,
10
]
]
},
"published-print": {
"date-parts": [
[
2002,
10
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1038/373117a0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_1_2"
},
{
"DOI": "10.1038/373123a0",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_2_2"
},
{
"DOI": "10.1056/NEJM199709113371101",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_3_2"
},
{
"DOI": "10.1056/NEJM199709113371102",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_4_2"
},
{
"DOI": "10.1016/S0140-6736(05)78643-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_5_2"
},
{
"DOI": "10.1016/S0140-6736(05)78643-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_6_2"
},
{
"DOI": "10.1016/S0140-6736(05)78644-X",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_7_2"
},
{
"DOI": "10.1016/S0002-9149(96)00159-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_8_2"
},
{
"DOI": "10.1111/1523-1747.ep12613748",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_9_2"
},
{
"DOI": "10.1083/jcb.144.5.903",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_10_2"
},
{
"DOI": "10.1073/pnas.94.2.514",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_11_2"
},
{
"DOI": "10.1016/S0021-9258(20)80645-0",
"doi-asserted-by": "crossref",
"first-page": "22042",
"journal-title": "J Biol Chem",
"key": "e_1_3_3_12_2",
"volume": "268",
"year": "1993"
},
{
"DOI": "10.1161/res.86.9.960",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_13_2"
},
{
"key": "e_1_3_3_14_2",
"unstructured": "Chen C Li JS Ren Z Chen X Ma M Conklin B Yao Y. HIV protease inhibitor ritonavir causes endothelial dysfunction in monkey arteries. Presented at: 11th Annual Meeting of The Society for Vascular Medicine and Biology; June 9–11 2000; Toronto Ontario Canada."
},
{
"DOI": "10.1111/j.1600-065X.1991.tb00822.x",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_15_2"
},
{
"first-page": "H165",
"journal-title": "Am J Physiol",
"key": "e_1_3_3_16_2",
"volume": "265",
"year": "1993"
},
{
"first-page": "110",
"journal-title": "Surg Forum",
"key": "e_1_3_3_17_2",
"volume": "80",
"year": "1994"
},
{
"key": "e_1_3_3_18_2",
"unstructured": "Physicians Desk Reference. 51st ed. Montvale NJ: Medical Economics-Thomson Healthcare; 1997: 447–451."
},
{
"DOI": "10.1001/jama.1997.03540260059037",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_19_2"
},
{
"DOI": "10.1056/NEJM199804303381808",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_20_2"
},
{
"DOI": "10.1016/S0140-6736(98)03391-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_21_2"
},
{
"DOI": "10.2165/00003088-199835040-00002",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_22_2"
},
{
"DOI": "10.1056/NEJM199810293391806",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_23_2"
},
{
"DOI": "10.1161/circ.100.7.700",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_24_2"
},
{
"DOI": "10.1097/00126334-200001010-00005",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_25_2"
},
{
"DOI": "10.1161/circ.101.9.948",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_26_2"
},
{
"DOI": "10.1161/circ.101.16.1899",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_27_2"
},
{
"DOI": "10.1016/S0002-9149(00)00857-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_28_2"
},
{
"DOI": "10.1016/S0002-9149(98)00702-4",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_29_2"
},
{
"DOI": "10.1161/circ.104.3.257",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_30_2"
},
{
"DOI": "10.1161/circ.97.25.2519",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_31_2"
},
{
"DOI": "10.1161/circ.96.10.3266",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_32_2"
},
{
"first-page": "1328.",
"journal-title": "Lancet",
"key": "e_1_3_3_33_2",
"volume": "351",
"year": "1998"
},
{
"first-page": "37",
"journal-title": "J HIV Ther",
"key": "e_1_3_3_34_2",
"volume": "6",
"year": "2001"
},
{
"DOI": "10.1086/317541",
"doi-asserted-by": "publisher",
"key": "e_1_3_3_35_2"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ahajournals.org/doi/10.1161/01.ATV.0000034707.40046.02"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cardiology and Cardiovascular Medicine"
],
"subtitle": [],
"title": "HIV Protease Inhibitor Ritonavir Induces Cytotoxicity of Human Endothelial Cells",
"type": "journal-article",
"volume": "22"
}