Identification of drugs associated with reduced severity of COVID-19: A case-control study in a large population
et al., Epidemiology and Global Health Microbiology and Infectious Disease, doi:10.7554/eLife.68165, Jul 2021
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
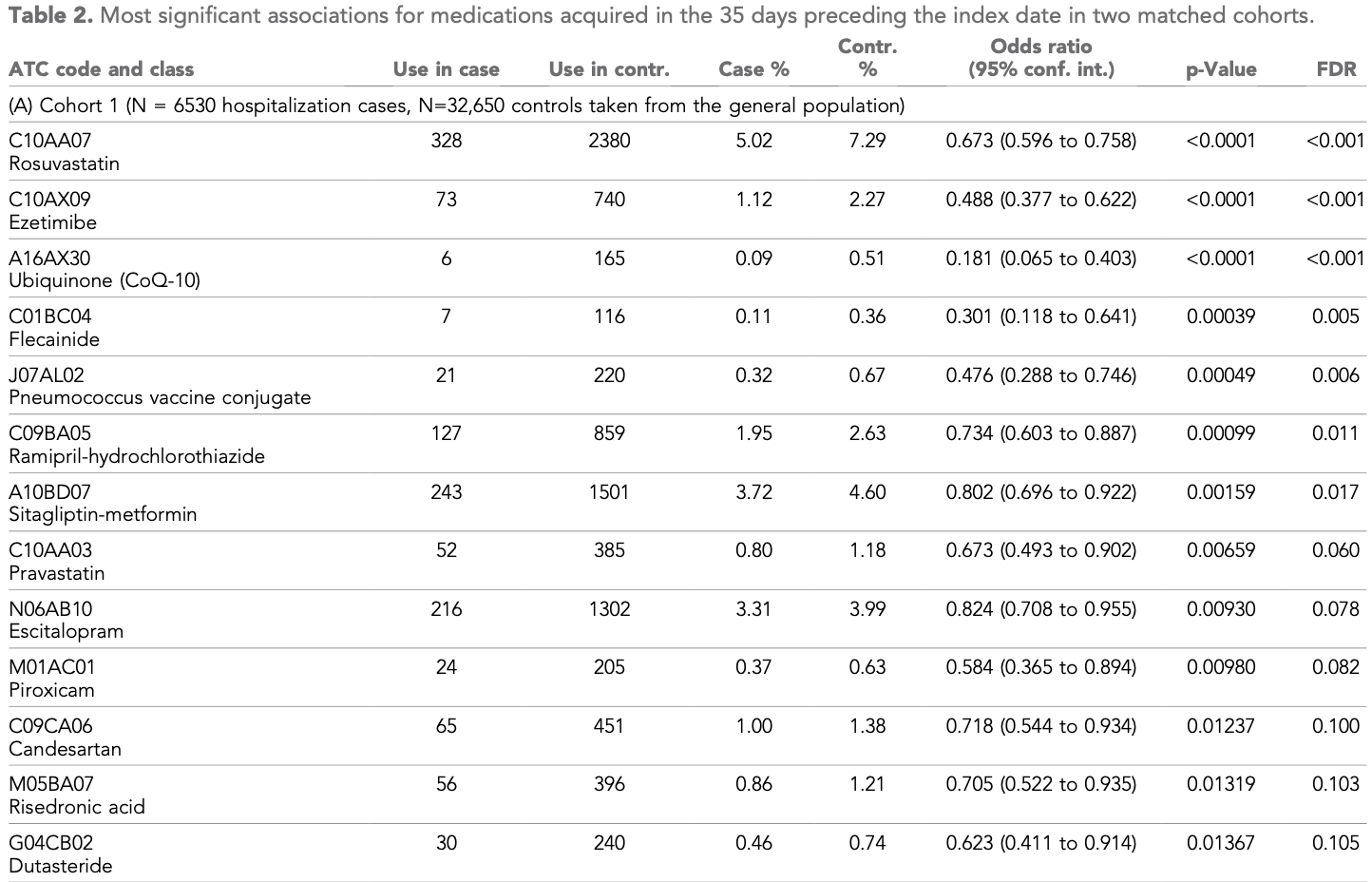
Case control study examining medication usage with a healthcare database in Israel, showing lower risk of hospitalization with dutasteride.
|
risk of hospitalization, 37.7% lower, OR 0.62, p = 0.01, treatment 30 of 6,530 (0.5%) cases,
240 of 32,650 (0.7%) controls, NNT 18, case control OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Israel et al., 27 Jul 2021, retrospective, Israel, peer-reviewed, 10 authors.
Identification of drugs associated with reduced severity of COVID-19 – a case-control study in a large population
eLife, doi:10.7554/elife.68165
Background: Until coronavirus disease 2019 (COVID-19) drugs specifically developed to treat COVID-19 become more widely accessible, it is crucial to identify whether existing medications have a protective effect against severe disease. Toward this objective, we conducted a large population study in Clalit Health Services (CHS), the largest healthcare provider in Israel, insuring over 4.7 million members. Methods: Two case-control matched cohorts were assembled to assess which medications, acquired in the last month, decreased the risk of COVID-19 hospitalization. Case patients were adults aged 18 to 95 hospitalized for COVID-19. In the first cohort, five control patients, from the general population, were matched to each case (n=6202); in the second cohort, two nonhospitalized SARS-CoV-2 positive control patients were matched to each case (n=6919). The outcome measures for a medication were: odds ratio (OR) for hospitalization, 95% confidence interval (CI), and the p-value, using Fisher's exact test. False discovery rate was used to adjust for multiple testing. Results: Medications associated with most significantly reduced odds for COVID-19 hospitalization include: ubiquinone (OR=0.185, 95% CI [0.058 to 0.458], p<0.001), ezetimibe (OR=0.488, 95% CI [0.377 to 0.622], p<0.001), rosuvastatin (OR=0.673, 95% CI [0.596 to 0.758], p<0.001), flecainide (OR=0.301, 95% CI [0.118 to 0.641], p<0.001), and vitamin D (OR=0.869, 95% CI [0.792 to 0.954], p<0.003). Remarkably, acquisition of artificial tears, eye care wipes, and several ophthalmological products were also associated with decreased risk for hospitalization. Conclusions: Ubiquinone, ezetimibe, and rosuvastatin, all related to the cholesterol synthesis pathway were associated with reduced hospitalization risk. These findings point to a promising protective effect which should be further investigated in controlled, prospective studies.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. Additional files
Author contributions
Supplementary files . Source code 1. R source code, producing Figure 1 . . Supplementary file 1. Supplementary tables and figures. Suppl Tab 1 Multivariable logistic regression for hospitalization status according to ethnicity and medication consumption in Cohort 1. Suppl Tab 2 Multivariable logistic regression for hospitalization status according to ethnicity and medication consumption in Cohort 2. Figure supplements Forest plot showing association between drug use and hospitalization risk in each of the cohorts, divided by body mass index (BMI) category.
Data availability Data were obtained from patients' electronic health records, and IRB approval restrains its use to researchers inside Clalit Health Services. For further information regarding data availability, researchers may contact Dr. Lavie gillav@clalit.org.il. This study is based on real-world patient drug purchases, and it cannot be made available due to patient privacy concerns. R code used to produce Figure 1 is available as a supplementary file named 'Source code 1'.
References
Aizaki, Morikawa, Fukasawa, Hara, Inoue et al., Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection, Journal of Virology, doi:10.1128/JVI.02530-07
Antonelli, Feldman, Freedberg, Darawsha, Rosenfeld, Intravenous flecainide administration for conversion of paroxysmal atrial fibrillation in the emergency room, Harefuah
Bajimaya, Hayashi, Frankl, Bryk, Ward et al., Cholesterol reducing agents inhibit assembly of type I parainfluenza viruses, Virology, doi:10.1016/j.virol.2016.11.011
Benjamini, Hochberg, Controlling the false discovery rate: a practical and powerful approach to multiple testing, Journal of the Royal Statistical Society: Series B
Bramante, Buse, Tamaritz, Palacio, Cohen et al., Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity, Journal of Medical Virology, doi:10.1002/jmv.26873
Buhaescu, Izzedine, Mevalonate pathway: a review of clinical and therapeutical implications, Clinical Biochemistry, doi:10.1016/j.clinbiochem.2007.03.016
Ci, Postema, Arbelo, Behr, Bezzina et al., SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes, Hear Rhythm, doi:10.1016/j.hrthm.2020.03.024
Dagliati, Malovini, Tibollo, Bellazzi, Health informatics and EHR to support clinical research in the COVID-19 pandemic: an overview, Briefings in Bioinformatics, doi:10.1093/bib/bbaa418
Estiri, Strasser, Klann, Naseri, Wagholikar et al., Predicting COVID-19 mortality with electronic medical records, Npj Digital Medicine, doi:10.1038/s41746-021-00383-x
Gower, Graham, Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro, Antimicrobial Agents and Chemotherapy, doi:10.1128/AAC.45.4.1231-1237.2001
Iroegbu, Ifenatuoha, Ijomone, Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2, Neurological Sciences, doi:10.1007/s10072-020-04469-4
Israel, Cicurel, Feldhamer, Dror, Giveon et al., The link between vitamin D deficiency and Covid-19 in a large population, medRxiv, doi:10.1101/2020.09.04.20188268
Kim, Ta, Liu, Sung, Butler et al., Towards clinical data-driven eligibility criteria optimization for interventional COVID-19 clinical trials, Journal of the American Medical Informatics Association, doi:10.1093/jamia/ocaa276
Koren, Shlezinger, Katz, Shalev, Amitai, Seawater desalination and serum magnesium concentrations in israel, Journal of Water and Health, doi:10.2166/wh.2016.164
Meinhardt, Radke, Dittmayer, Franz, Thomas et al., Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19, Nature Neuroscience, doi:10.1038/s41593-020-00758-5
Merzon, Tworowski, Gorohovski, Vinker, Cohen et al., Low plasma 25(OH) vitamin D level is associated with increased risk of covid-19 infection: an israeli population-based study, The FEBS Journal, doi:10.1111/febs.15495
Morales, Conover, You, Pratt, Kostka et al., Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis, The Lancet Digital Health, doi:10.1016/S2589-7500(20)30289-2
Muhsen, Lapidot, Goren, Amir, Perlman et al., A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, The Lancet Regional Health -Europe, doi:10.1016/j.lanepe.2021.100130
Osborne, Veigulis, Arreola, ¨o ¨sli, Curtin, Automated EHR score to predict COVID-19 outcomes at US department of veterans affairs, PLOS ONE, doi:10.1371/journal.pone.0236554
Osuna-Ramos, Reyes-Ruiz, ´ngel, The role of host cholesterol during Flavivirus infection, Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2018.00388
Qu, Guo, Chai, Wang, Gao et al., Effects of coenzyme Q10 on Statin-Induced myopathy: an updated Meta-Analysis of randomized controlled trials, Journal of the American Heart Association, doi:10.1161/JAHA.118.009835
Ragan, Hartson, Pidcoke, Bowen, Goodrich, Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using Riboflavin and UV light, PLOS ONE, doi:10.1371/journal.pone.0233947
Rossman, Jing, Leser, Balannik, Pinto et al., Influenza virus m2 ion channel protein is necessary for filamentous virion formation, Journal of Virology, doi:10.1128/JVI.00119-10
Shadmi, Balicer, Kinder, Abrams, Weiner, Assessing socioeconomic health care utilization inequity in Israel: impact of alternative approaches to morbidity adjustment, BMC Public Health, doi:10.1186/1471-2458-11-609
Song, Zhang, Israelow, Lu-Culligan, Prado et al., Neuroinvasion of SARS-CoV-2 in human and mouse brain, Journal of Experimental Medicine, doi:10.1084/jem.20202135
Sudat, Robinson, Mudiganti, Mani, Pressman, Mind the clinical-analytic gap: electronic health records and COVID-19 pandemic response, Journal of Biomedical Informatics, doi:10.1016/j.jbi.2021.103715
Sun, Whittaker, Role for influenza virus envelope cholesterol in virus entry and infection, Journal of Virology, doi:10.1128/JVI.77.23.12543-12551.2003
Trasino, A role for retinoids in the treatment of COVID-19?, Clinical and Experimental Pharmacology and Physiology, doi:10.1111/1440-1681.13354
Tsoumpra, Muniz, Barnett, Kwaasi, Pilka et al., The inhibition of human farnesyl pyrophosphate synthase by nitrogen-containing bisphosphonates. Elucidating the role of active site threonine 201 and tyrosine 204 residues using enzyme mutants, Bone, doi:10.1016/j.bone.2015.08.020
Uwitonze, Razzaque, Role of magnesium in vitamin D activation and function, The Journal of the American Osteopathic Association, doi:10.7556/jaoa.2018.037
Wagner, Shweta, Murugadoss, Awasthi, Venkatakrishnan et al., Augmented curation of clinical notes from a massive EHR system reveals symptoms of impending COVID-19 diagnosis, eLife, doi:10.7554/eLife.58227
Wessels, Rolles, Rink, The potential impact of zinc supplementation on COVID-19 pathogenesis, Frontiers in Immunology, doi:10.3389/fimmu.2020.01712
Whitehorn, Nguyen, Khanh, Kien, Quyen et al., Lovastatin for the treatment of adult patients with dengue: a randomized, Double-Blind, Placebo-Controlled trial, Clinical Infectious Diseases, doi:10.1093/cid/civ949
Yavuz, Ertugrul, Cil, Ata, Akin et al., Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins?, Cardiovascular Drugs and Therapy, doi:10.1007/s10557-009-6181-8
Yavuz, Ertugrul, Statins and vitamin D: a hot topic that will be discussed for a long time, Dermato-Endocrinology, doi:10.4161/derm.20188
Zeng, Wang, Li, Yang, Qiu et al., Association of daily wear of eyeglasses with susceptibility to coronavirus disease 2019 infection, JAMA Ophthalmology, doi:10.1001/jamaophthalmol.2020.3906
DOI record:
{
"DOI": "10.7554/elife.68165",
"ISSN": [
"2050-084X"
],
"URL": "http://dx.doi.org/10.7554/eLife.68165",
"abstract": "<jats:sec id=\"abs1\"><jats:title>Background:</jats:title><jats:p>Until coronavirus disease 2019 (COVID-19) drugs specifically developed to treat COVID-19 become more widely accessible, it is crucial to identify whether existing medications have a protective effect against severe disease. Toward this objective, we conducted a large population study in Clalit Health Services (CHS), the largest healthcare provider in Israel, insuring over 4.7 million members.</jats:p></jats:sec><jats:sec id=\"abs2\"><jats:title>Methods:</jats:title><jats:p>Two case-control matched cohorts were assembled to assess which medications, acquired in the last month, decreased the risk of COVID-19 hospitalization. Case patients were adults aged 18 to 95 hospitalized for COVID-19. In the first cohort, five control patients, from the general population, were matched to each case (n=6202); in the second cohort, two non-hospitalized SARS-CoV-2 positive control patients were matched to each case (n=6919). The outcome measures for a medication were: odds ratio (OR) for hospitalization, 95% confidence interval (CI), and the p-value, using Fisher’s exact test. False discovery rate was used to adjust for multiple testing.</jats:p></jats:sec><jats:sec id=\"abs3\"><jats:title>Results:</jats:title><jats:p>Medications associated with most significantly reduced odds for COVID-19 hospitalization include: ubiquinone (OR=0.185, 95% CI [0.058 to 0.458], p<0.001), ezetimibe (OR=0.488, 95% CI [0.377 to 0.622], p<0.001), rosuvastatin (OR=0.673, 95% CI [0.596 to 0.758], p<0.001), flecainide (OR=0.301, 95% CI [0.118 to 0.641], p<0.001), and vitamin D (OR=0.869, 95% CI [0.792 to 0.954], p<0.003). Remarkably, acquisition of artificial tears, eye care wipes, and several ophthalmological products were also associated with decreased risk for hospitalization.</jats:p></jats:sec><jats:sec id=\"abs4\"><jats:title>Conclusions:</jats:title><jats:p>Ubiquinone, ezetimibe, and rosuvastatin, all related to the cholesterol synthesis pathway were associated with reduced hospitalization risk. These findings point to a promising protective effect which should be further investigated in controlled, prospective studies.</jats:p></jats:sec><jats:sec id=\"abs5\"><jats:title>Funding:</jats:title><jats:p>This research was supported in part by the Intramural Research Program of the National Institutes of Health, NCI.</jats:p></jats:sec>",
"alternative-id": [
"10.7554/eLife.68165"
],
"assertion": [
{
"group": {
"name": "peer_review_taxonomy"
},
"label": "Peer review transparency",
"name": "peer_review_transparency",
"value": "single anonymised"
},
{
"group": {
"name": "peer_review_taxonomy"
},
"label": "Peer review interaction",
"name": "peer_review_interaction",
"value": "other reviewer(s), editor"
},
{
"group": {
"name": "peer_review_taxonomy"
},
"label": "Peer review published",
"name": "peer_review_published",
"value": "review summaries, review reports, author/editor communication, reviewer identities reviewer opt in, editor identities"
},
{
"group": {
"name": "post_publication_commenting"
},
"label": "Post publication commenting",
"name": "post_publication_commenting",
"value": "open (sign in with ORCID iD required)"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4389-8896",
"affiliation": [
{
"name": "Division of Planning and Strategy, Clalit Health Services, Tel Aviv, Israel"
}
],
"authenticated-orcid": true,
"family": "Israel",
"given": "Ariel",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2147-8033",
"affiliation": [
{
"name": "Cancer Data Science Laboratory, National Cancer Institute, National Institutes of Health, Bethesda, United States"
}
],
"authenticated-orcid": true,
"family": "Schäffer",
"given": "Alejandro A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Planning and Strategy, Clalit Health Services, Tel Aviv, Israel"
},
{
"name": "Clalit Health Services, Southern District and Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel"
}
],
"family": "Cicurel",
"given": "Assi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Data Science Laboratory, National Cancer Institute, National Institutes of Health, Bethesda, United States"
}
],
"family": "Cheng",
"given": "Kuoyuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Data Science Laboratory, National Cancer Institute, National Institutes of Health, Bethesda, United States"
}
],
"family": "Sinha",
"given": "Sanju",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sheba Medical Center, Tel-Aviv University, Ramat Gan, Israel"
}
],
"family": "Schiff",
"given": "Eyal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Planning and Strategy, Clalit Health Services, Tel Aviv, Israel"
}
],
"family": "Feldhamer",
"given": "Ilan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Planning and Strategy, Clalit Health Services, Tel Aviv, Israel"
}
],
"family": "Tal",
"given": "Ameer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Planning and Strategy, Clalit Health Services, Tel Aviv, Israel"
},
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion – Israel Institute of Technology, Haifa, Israel"
}
],
"family": "Lavie",
"given": "Gil",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7862-3940",
"affiliation": [
{
"name": "Cancer Data Science Laboratory, National Cancer Institute, National Institutes of Health, Bethesda, United States"
}
],
"authenticated-orcid": true,
"family": "Ruppin",
"given": "Eytan",
"sequence": "additional"
}
],
"container-title": "eLife",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"elifesciences.org"
]
},
"created": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T12:00:27Z",
"timestamp": 1627387227000
},
"deposited": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T01:14:53Z",
"timestamp": 1697073293000
},
"funder": [
{
"DOI": "10.13039/100000054",
"award": [
"Intramural funding"
],
"doi-asserted-by": "publisher",
"name": "National Cancer Institute"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
1
]
],
"date-time": "2023-12-01T11:33:49Z",
"timestamp": 1701430429975
},
"is-referenced-by-count": 24,
"issued": {
"date-parts": [
[
2021,
7,
27
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/publicdomain/zero/1.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T00:00:00Z",
"timestamp": 1627344000000
}
},
{
"URL": "http://creativecommons.org/publicdomain/zero/1.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T00:00:00Z",
"timestamp": 1627344000000
}
},
{
"URL": "http://creativecommons.org/publicdomain/zero/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T00:00:00Z",
"timestamp": 1627344000000
}
}
],
"link": [
{
"URL": "https://cdn.elifesciences.org/articles/68165/elife-68165-v2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://cdn.elifesciences.org/articles/68165/elife-68165-v2.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://elifesciences.org/articles/68165",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4374",
"original-title": [],
"prefix": "10.7554",
"published": {
"date-parts": [
[
2021,
7,
27
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
27
]
]
},
"publisher": "eLife Sciences Publications, Ltd",
"reference": [
{
"DOI": "10.1128/JVI.02530-07",
"article-title": "Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection",
"author": "Aizaki",
"doi-asserted-by": "publisher",
"first-page": "5715",
"journal-title": "Journal of Virology",
"key": "bib1",
"volume": "82",
"year": "2008"
},
{
"article-title": "Intravenous flecainide administration for conversion of paroxysmal atrial fibrillation in the emergency room",
"author": "Antonelli",
"first-page": "342",
"journal-title": "Harefuah",
"key": "bib2",
"volume": "145",
"year": "2006"
},
{
"DOI": "10.1016/j.virol.2016.11.011",
"article-title": "Cholesterol reducing agents inhibit assembly of type I parainfluenza viruses",
"author": "Bajimaya",
"doi-asserted-by": "publisher",
"first-page": "127",
"journal-title": "Virology",
"key": "bib3",
"volume": "501",
"year": "2017"
},
{
"article-title": "Controlling the false discovery rate: a practical and powerful approach to multiple testing",
"author": "Benjamini",
"first-page": "289",
"journal-title": "Journal of the Royal Statistical Society: Series B",
"key": "bib4",
"volume": "57",
"year": "1995"
},
{
"DOI": "10.1002/jmv.26873",
"article-title": "Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity",
"author": "Bramante",
"doi-asserted-by": "publisher",
"first-page": "4273",
"journal-title": "Journal of Medical Virology",
"key": "bib5",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.clinbiochem.2007.03.016",
"article-title": "Mevalonate pathway: a review of clinical and therapeutical implications",
"author": "Buhaescu",
"doi-asserted-by": "publisher",
"first-page": "575",
"journal-title": "Clinical Biochemistry",
"key": "bib6",
"volume": "40",
"year": "2007"
},
{
"DOI": "10.1016/j.hrthm.2020.03.024",
"article-title": "SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes",
"author": "Ci",
"doi-asserted-by": "publisher",
"first-page": "1456",
"journal-title": "Hear Rhythm",
"key": "bib7",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1093/bib/bbaa418",
"article-title": "Health informatics and EHR to support clinical research in the COVID-19 pandemic: an overview",
"author": "Dagliati",
"doi-asserted-by": "publisher",
"first-page": "812",
"journal-title": "Briefings in Bioinformatics",
"key": "bib8",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1038/s41746-021-00383-x",
"article-title": "Predicting COVID-19 mortality with electronic medical records",
"author": "Estiri",
"doi-asserted-by": "publisher",
"journal-title": "Npj Digital Medicine",
"key": "bib9",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1128/AAC.45.4.1231-1237.2001",
"article-title": "Antiviral activity of lovastatin against respiratory syncytial virus in vivo and in vitro",
"author": "Gower",
"doi-asserted-by": "publisher",
"first-page": "1231",
"journal-title": "Antimicrobial Agents and Chemotherapy",
"key": "bib10",
"volume": "45",
"year": "2001"
},
{
"DOI": "10.1007/s10072-020-04469-4",
"article-title": "Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2",
"author": "Iroegbu",
"doi-asserted-by": "publisher",
"first-page": "1329",
"journal-title": "Neurological Sciences",
"key": "bib11",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1101/2020.09.04.20188268",
"article-title": "The link between vitamin D deficiency and Covid-19 in a large population",
"author": "Israel",
"doi-asserted-by": "publisher",
"key": "bib12",
"volume-title": "medRxiv",
"year": "2020"
},
{
"DOI": "10.1093/jamia/ocaa276",
"article-title": "Towards clinical data-driven eligibility criteria optimization for interventional COVID-19 clinical trials",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "14",
"journal-title": "Journal of the American Medical Informatics Association",
"key": "bib13",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.2166/wh.2016.164",
"article-title": "Seawater desalination and serum magnesium concentrations in israel",
"author": "Koren",
"doi-asserted-by": "publisher",
"first-page": "296",
"journal-title": "Journal of Water and Health",
"key": "bib14",
"volume": "15",
"year": "2017"
},
{
"DOI": "10.1038/s41593-020-00758-5",
"article-title": "Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19",
"author": "Meinhardt",
"doi-asserted-by": "publisher",
"first-page": "168",
"journal-title": "Nature Neuroscience",
"key": "bib15",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1111/febs.15495",
"article-title": "Low plasma 25(OH) vitamin D level is associated with increased risk of covid‐19 infection: an israeli population‐based study",
"author": "Merzon",
"doi-asserted-by": "publisher",
"first-page": "3693",
"journal-title": "The FEBS Journal",
"key": "bib16",
"volume": "287",
"year": "2020"
},
{
"DOI": "10.1016/S2589-7500(20)30289-2",
"article-title": "Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis",
"author": "Morales",
"doi-asserted-by": "publisher",
"first-page": "e98",
"journal-title": "The Lancet Digital Health",
"key": "bib17",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/j.lanepe.2021.100130",
"article-title": "A nationwide analysis of population group differences in the COVID-19 epidemic in Israel, February 2020-February 2021",
"author": "Muhsen",
"doi-asserted-by": "publisher",
"journal-title": "The Lancet Regional Health - Europe",
"key": "bib18",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0236554",
"article-title": "Automated EHR score to predict COVID-19 outcomes at US department of veterans affairs",
"author": "Osborne",
"doi-asserted-by": "publisher",
"journal-title": "PLOS ONE",
"key": "bib19",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3389/fcimb.2018.00388",
"article-title": "The role of host cholesterol during Flavivirus infection",
"author": "Osuna-Ramos",
"doi-asserted-by": "publisher",
"journal-title": "Frontiers in Cellular and Infection Microbiology",
"key": "bib20",
"volume": "8",
"year": "2018"
},
{
"DOI": "10.1161/JAHA.118.009835",
"article-title": "Effects of coenzyme Q10 on Statin-Induced myopathy: an updated Meta-Analysis of randomized controlled trials",
"author": "Qu",
"doi-asserted-by": "publisher",
"journal-title": "Journal of the American Heart Association",
"key": "bib21",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0233947",
"article-title": "Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using Riboflavin and UV light",
"author": "Ragan",
"doi-asserted-by": "publisher",
"journal-title": "PLOS ONE",
"key": "bib22",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1128/JVI.00119-10",
"article-title": "Influenza virus m2 ion channel protein is necessary for filamentous virion formation",
"author": "Rossman",
"doi-asserted-by": "publisher",
"first-page": "5078",
"journal-title": "Journal of Virology",
"key": "bib23",
"volume": "84",
"year": "2010"
},
{
"DOI": "10.1186/1471-2458-11-609",
"article-title": "Assessing socioeconomic health care utilization inequity in Israel: impact of alternative approaches to morbidity adjustment",
"author": "Shadmi",
"doi-asserted-by": "publisher",
"journal-title": "BMC Public Health",
"key": "bib24",
"volume": "11",
"year": "2011"
},
{
"DOI": "10.1084/jem.20202135",
"article-title": "Neuroinvasion of SARS-CoV-2 in human and mouse brain",
"author": "Song",
"doi-asserted-by": "publisher",
"journal-title": "Journal of Experimental Medicine",
"key": "bib25",
"volume": "218",
"year": "2021"
},
{
"DOI": "10.1016/j.jbi.2021.103715",
"article-title": "Mind the clinical-analytic gap: electronic health records and COVID-19 pandemic response",
"author": "Sudat",
"doi-asserted-by": "publisher",
"journal-title": "Journal of Biomedical Informatics",
"key": "bib26",
"volume": "116",
"year": "2021"
},
{
"DOI": "10.1128/JVI.77.23.12543-12551.2003",
"article-title": "Role for influenza virus envelope cholesterol in virus entry and infection",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "12543",
"journal-title": "Journal of Virology",
"key": "bib27",
"volume": "77",
"year": "2003"
},
{
"DOI": "10.1111/1440-1681.13354",
"article-title": "A role for retinoids in the treatment of COVID-19?",
"author": "Trasino",
"doi-asserted-by": "publisher",
"first-page": "1765",
"journal-title": "Clinical and Experimental Pharmacology and Physiology",
"key": "bib28",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1016/j.bone.2015.08.020",
"article-title": "The inhibition of human farnesyl pyrophosphate synthase by nitrogen-containing bisphosphonates. Elucidating the role of active site threonine 201 and tyrosine 204 residues using enzyme mutants",
"author": "Tsoumpra",
"doi-asserted-by": "publisher",
"first-page": "478",
"journal-title": "Bone",
"key": "bib29",
"volume": "81",
"year": "2015"
},
{
"DOI": "10.7556/jaoa.2018.037",
"article-title": "Role of magnesium in vitamin D activation and function",
"author": "Uwitonze",
"doi-asserted-by": "publisher",
"first-page": "181",
"journal-title": "The Journal of the American Osteopathic Association",
"key": "bib30",
"volume": "118",
"year": "2018"
},
{
"DOI": "10.7554/eLife.58227",
"article-title": "Augmented curation of clinical notes from a massive EHR system reveals symptoms of impending COVID-19 diagnosis",
"author": "Wagner",
"doi-asserted-by": "publisher",
"journal-title": "eLife",
"key": "bib31",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01712",
"article-title": "The potential impact of zinc supplementation on COVID-19 pathogenesis",
"author": "Wessels",
"doi-asserted-by": "publisher",
"journal-title": "Frontiers in Immunology",
"key": "bib32",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/cid/civ949",
"article-title": "Lovastatin for the treatment of adult patients with dengue: a randomized, Double-Blind, Placebo-Controlled trial",
"author": "Whitehorn",
"doi-asserted-by": "publisher",
"journal-title": "Clinical Infectious Diseases",
"key": "bib33",
"volume": "62",
"year": "2016"
},
{
"DOI": "10.1007/s10557-009-6181-8",
"article-title": "Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins?",
"author": "Yavuz",
"doi-asserted-by": "publisher",
"first-page": "295",
"journal-title": "Cardiovascular Drugs and Therapy",
"key": "bib34",
"volume": "23",
"year": "2009"
},
{
"DOI": "10.4161/derm.20188",
"article-title": "Statins and vitamin D: a hot topic that will be discussed for a long time",
"author": "Yavuz",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Dermato-Endocrinology",
"key": "bib35",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.1001/jamaophthalmol.2020.3906",
"article-title": "Association of daily wear of eyeglasses with susceptibility to coronavirus disease 2019 infection",
"author": "Zeng",
"doi-asserted-by": "publisher",
"first-page": "1196",
"journal-title": "JAMA Ophthalmology",
"key": "bib36",
"volume": "138",
"year": "2020"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {
"has-preprint": [
{
"asserted-by": "subject",
"id": "10.1101/2020.10.13.20211953",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://elifesciences.org/articles/68165"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Immunology and Microbiology",
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine",
"General Neuroscience"
],
"subtitle": [],
"title": "Identification of drugs associated with reduced severity of COVID-19 – a case-control study in a large population",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.7554/elife.68165",
"volume": "10"
}
israel2
