Results from a Phase 1 First in Human Study of Pemivibart: An Extended Half-Life Monoclonal Antibody (mAb)
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofae631.2185, Jan 2025
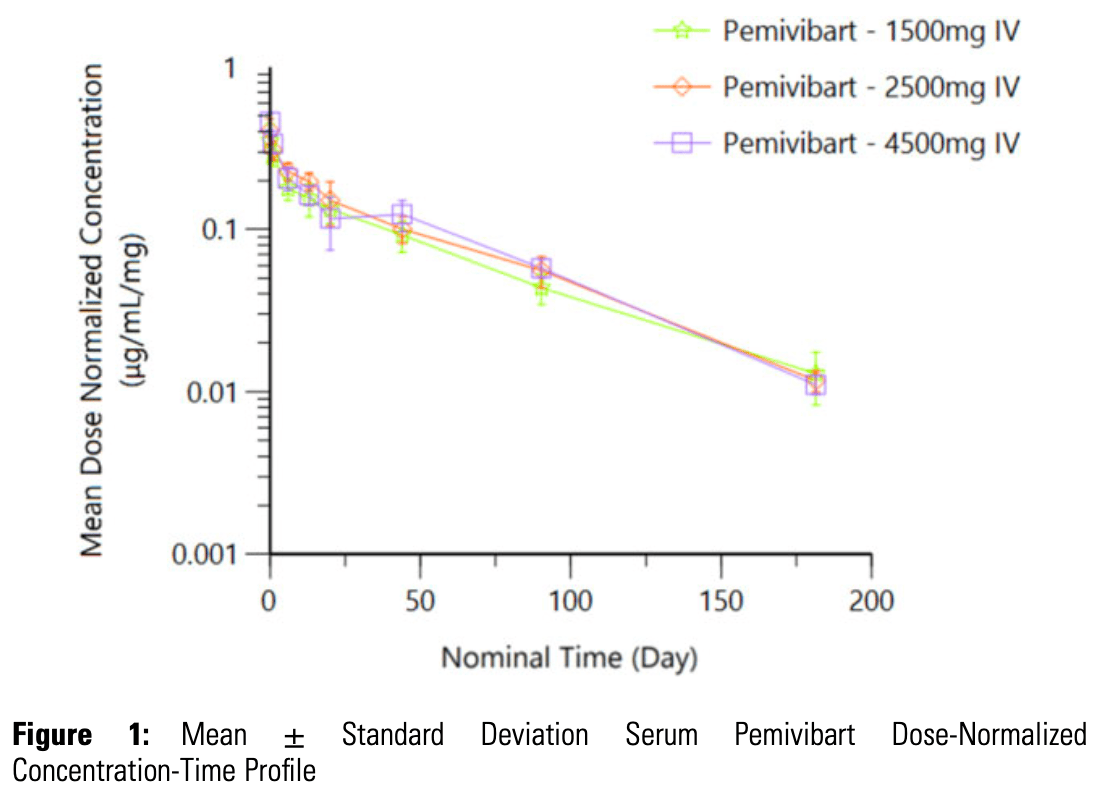
Phase 1 RCT with 30 healthy participants showing pemivibart was well-tolerated at doses up to 4500 mg, with no serious adverse events or adverse events leading to drug discontinuation reported. Pemivibart demonstrated linear and dose-proportional pharmacokinetics with an extended serum half-life of 44-50 days.
Efficacy is variant dependent. In Vitro research shows reduced efficacy against KP.3.1.1, KP.1.1, LB.1, KP.3.3, and XEC variants1-4.
1.
Xie et al., Molecular Basis of High-Blood-Pressure-Enhanced and High-Fever-Temperature-Weakened Receptor-Binding Domain/Peptidase Domain Binding: A Molecular Dynamics Simulation Study, International Journal of Molecular Sciences, doi:10.3390/ijms26073250.
2.
Wang et al., Activity of Research-Grade Pemivibart against Recent SARS-CoV-2 JN.1 Sublineages, New England Journal of Medicine, doi:10.1056/NEJMc2410203.
Holmes et al., 29 Jan 2025, Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, median age 32.5, 9 authors.
Abstract: P-2029. Results from a Phase 1 First in Human Study of Pemivibart: An Extended
Half-Life Monoclonal Antibody (mAb)
Anna Holmes, PhD1; Yong Li, PhD1; Deepali Gupta, BSc1; Ed Campanaro, MS1;
Lida Mehr, B.S.2; Anuja Raut, M.S.3; Amanda Copans, Pharm.D.1; Kristin Narayan,
Ph.D.1; Kathryn Mahoney, PharmD1; 1Invivyd, Inc., Waltham, MA; 2Lida Mehr
Consulting, LLC, MIssion Viejo, California; 3Certera, Inc., Radnor, Pennsylvania
Study Group:
Session: 226. COVID-19: Treatment
Saturday, October 19, 2024: 12:15 PM
Background. Pemivibart (PEM) is a half-life extended recombinant human
monoclonal IgG1 antibody that targets the SARS-CoV-2 spike protein receptor binding domain. PEM has been granted emergency use authorization (EUA) for the preexposure prophylaxis of COVID-19 in certain adults and adolescents with
moderate-to-severe immune compromise.
Table 1.
1
1
3
1 1
2
3
1
3
Shionogi&Co., Ltd., Toyonaka, Osaka, Japan 2SHIONOGI
& CO., LTD., Toyonaka-shi, Osaka, Japan 3Shionogi TechnoAdvance Research, Co.,
Ltd., Toyonaka, Osaka, Japan
Preliminary Pharmacokinetic Parameter Estimates of Pemivibart [Mean ± SD]
Methods. This is an ongoing Phase 1, first in human, randomized, blinded, placebo (PBO) controlled, single ascending dose study conducted in healthy participants
(ppts) aged 18-65 years to evaluate PEM administered by slow IV push. Ppts were randomized 8:2 in one of 3 cohorts (n=8 PEM, n=2 placebo): PEM 1500 mg, 2500 mg,
and 4500 mg. The primary objective was to assess the safety and tolerability of
PEM. Secondary endpoints included serum pharmacokinetic (PK) parameters (estimated using non-compartmental analysis methodology) and immunogenicity. The
Poster Abstracts • OFID 2025:12 (Suppl 1) • S1205
Abstract citation ID: ofae631.2185
trial will continue through 12 months; here we summarize the data through the
6-month timepoint.
Deviation
Serum
Pemivibart
Dose-Normalized
Results. Thirty ppts were randomized (24 PEM, 6 PBO); 28 ppts received the full
dose as planned; two ppts received approximately 93% of the full dose of study drug
due to an administration error. In the PEM arm, the median age was 32.5 years, 13.3%
of ppts were 55 years or older, most ppts were white (83.3%) and not Hispanic or
Latinx (93.3%). Mean body mass index was 25.78 kg/m2 across all ppts and similar between cohorts and study arms. There were no deaths, serious adverse events (SAEs), or
AEs leading to permanent study drug discontinuation. Infusion-related adverse events
occurred in 4 ppts (2 each in Cohort 2 and 3, respectively); these events were selflimited and resolved within 5 minutes without treatment. No unexpected safety signals were observed. PEM demonstrated linear PK with apparent dose-proportional
exposure and extended serum half-life (mean 46, 44, and 50 days in Cohort 1, 2,
and 3, respectively [Table 1]). No substantial anti-drug antibodies (ADAs) have
been observed.
Conclusion. In this Phase 1 study of PEM administered by slow IV push at doses
up to 4500 mg, no AEs leading to study drug discontinuation, or SAEs were reported
to date in healthy adults. PEM demonstrated linear and dose-proportional PK.
Complete trial data to be presented.
Disclosures. Anna Holmes, PhD, Invivyd, Inc.: employee|Invivyd, Inc.: Stocks/
Bonds (Private Company) Yong Li, PhD, Invivyd, Inc.: Employee|Invivyd, Inc.:
Stocks/Bonds (Public Company) Deepali..
DOI record:
{
"DOI": "10.1093/ofid/ofae631.2185",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofae631.2185",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Pemivibart (PEM) is a half-life extended recombinant human monoclonal IgG1 antibody that targets the SARS-CoV-2 spike protein receptor binding domain. PEM has been granted emergency use authorization (EUA) for the pre-exposure prophylaxis of COVID-19 in certain adults and adolescents with moderate-to-severe immune compromise.Table 1.Preliminary Pharmacokinetic Parameter Estimates of Pemivibart [Mean ± SD]</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This is an ongoing Phase 1, first in human, randomized, blinded, placebo (PBO) controlled, single ascending dose study conducted in healthy participants (ppts) aged 18-65 years to evaluate PEM administered by slow IV push. Ppts were randomized 8:2 in one of 3 cohorts (n=8 PEM, n=2 placebo): PEM 1500 mg, 2500 mg, and 4500 mg. The primary objective was to assess the safety and tolerability of PEM. Secondary endpoints included serum pharmacokinetic (PK) parameters (estimated using non-compartmental analysis methodology) and immunogenicity. The trial will continue through 12 months; here we summarize the data through the 6-month timepoint.Figure 1:Mean ± Standard Deviation Serum Pemivibart Dose-Normalized Concentration-Time Profile</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Thirty ppts were randomized (24 PEM, 6 PBO); 28 ppts received the full dose as planned; two ppts received approximately 93% of the full dose of study drug due to an administration error. In the PEM arm, the median age was 32.5 years, 13.3% of ppts were 55 years or older, most ppts were white (83.3%) and not Hispanic or Latinx (93.3%). Mean body mass index was 25.78 kg/m2 across all ppts and similar between cohorts and study arms. There were no deaths, serious adverse events (SAEs), or AEs leading to permanent study drug discontinuation. Infusion-related adverse events occurred in 4 ppts (2 each in Cohort 2 and 3, respectively); these events were self-limited and resolved within 5 minutes without treatment. No unexpected safety signals were observed. PEM demonstrated linear PK with apparent dose-proportional exposure and extended serum half-life (mean 46, 44, and 50 days in Cohort 1, 2, and 3, respectively [Table 1]). No substantial anti-drug antibodies (ADAs) have been observed.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>In this Phase 1 study of PEM administered by slow IV push at doses up to 4500 mg, no AEs leading to study drug discontinuation, or SAEs were reported to date in healthy adults. PEM demonstrated linear and dose-proportional PK. Complete trial data to be presented.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Disclosures</jats:title>\n <jats:p>Anna Holmes, PhD, Invivyd, Inc.: employee|Invivyd, Inc.: Stocks/Bonds (Private Company) Yong Li, PhD, Invivyd, Inc.: Employee|Invivyd, Inc.: Stocks/Bonds (Public Company) Deepali Gupta, BSc, Invivyd, Inc.: Employee|Invivyd, Inc.: Stocks/Bonds (Public Company) Ed Campanaro, MS, Invivyd, Inc.: Employee|Invivyd, Inc.: Stocks/Bonds (Public Company) Lida Mehr, B.S., Invivyd, Inc.: Advisor/Consultant Anuja Raut, M.S., Invivyd, Inc.: Advisor/Consultant Amanda Copans, Pharm.D., Invivyd, Inc.: Employee|Invivyd, Inc.: Stocks/Bonds (Public Company) Kristin Narayan, Ph.D., Invivyd, Inc.: Employee|Invivyd, Inc.: Stocks/Bonds (Public Company) Kathryn Mahoney, PharmD, Invivyd, Inc.: Employee|Invivyd, Inc.: Stocks/Bonds (Public Company)</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Holmes",
"given": "Anna",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Li",
"given": "Yong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Gupta",
"given": "Deepali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Campanaro",
"given": "Ed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Lida Mehr Consulting, LLC , MIssion Viejo, California"
}
],
"family": "Mehr",
"given": "Lida",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Certera, Inc. , Radnor, Pennsylvania"
}
],
"family": "Raut",
"given": "Anuja",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Copans",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Narayan",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, MA"
}
],
"family": "Mahoney",
"given": "Kathryn",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
1,
29
]
],
"date-time": "2025-01-29T20:42:47Z",
"timestamp": 1738183367000
},
"deposited": {
"date-parts": [
[
2025,
1,
29
]
],
"date-time": "2025-01-29T22:37:37Z",
"timestamp": 1738190257000
},
"indexed": {
"date-parts": [
[
2025,
1,
30
]
],
"date-time": "2025-01-30T06:18:37Z",
"timestamp": 1738217917240,
"version": "3.34.0"
},
"is-referenced-by-count": 0,
"issue": "Supplement_1",
"issued": {
"date-parts": [
[
2025,
1,
29
]
]
},
"journal-issue": {
"issue": "Supplement_1",
"published-print": {
"date-parts": [
[
2025,
1,
29
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
29
]
],
"date-time": "2025-01-29T00:00:00Z",
"timestamp": 1738108800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/article-pdf/12/Supplement_1/ofae631.2185/61681463/ofae631.2185.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/12/Supplement_1/ofae631.2185/61681463/ofae631.2185.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2025,
1,
29
]
]
},
"published-online": {
"date-parts": [
[
2025,
1,
29
]
]
},
"published-other": {
"date-parts": [
[
2025,
2
]
]
},
"published-print": {
"date-parts": [
[
2025,
1,
29
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofae631.2185/7989146"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "P-2029. Results from a Phase 1 First in Human Study of Pemivibart: An Extended Half-Life Monoclonal Antibody (mAb)",
"type": "journal-article",
"volume": "12"
}