The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial
et al., Immunopathologia Persa, doi:10.34172/ipp.2022.08, Apr 2021
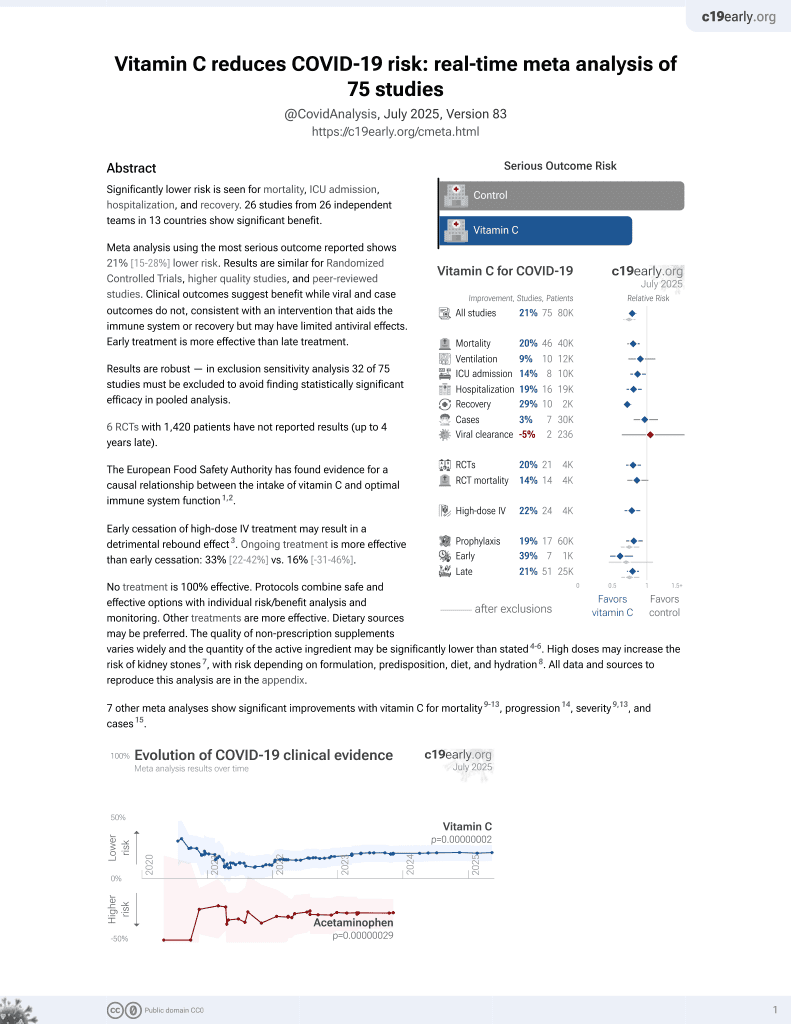
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT with 38 patients treated with vitamin C and vitamin E, and 34 control patients, showing lower ICU admission with treatment, but not statistically significant.
This is the 6th of 20 COVID-19 RCTs for vitamin C, which collectively show efficacy with p=0.0016.
This is the 21st of 73 COVID-19 controlled studies for vitamin C, which collectively show efficacy with p=0.000000076.
|
risk of ICU admission, 46.3% lower, RR 0.54, p = 0.46, treatment 3 of 38 (7.9%), control 5 of 34 (14.7%), NNT 15.
|
|
hospitalization time, 1.0% lower, relative time 0.99, p = 0.82, treatment 38, control 34.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Hakamifard et al., 14 Apr 2021, Randomized Controlled Trial, Iran, peer-reviewed, 8 authors, study period March 2020 - April 2020, dosage 1000mg daily, this trial uses multiple treatments in the treatment arm (combined with vitamin E) - results of individual treatments may vary.
The effect of vitamin E and vitamin C in patients with COVID-19 pneumonia; a randomized controlled clinical trial
doi:10.34172/ipp.2022.08
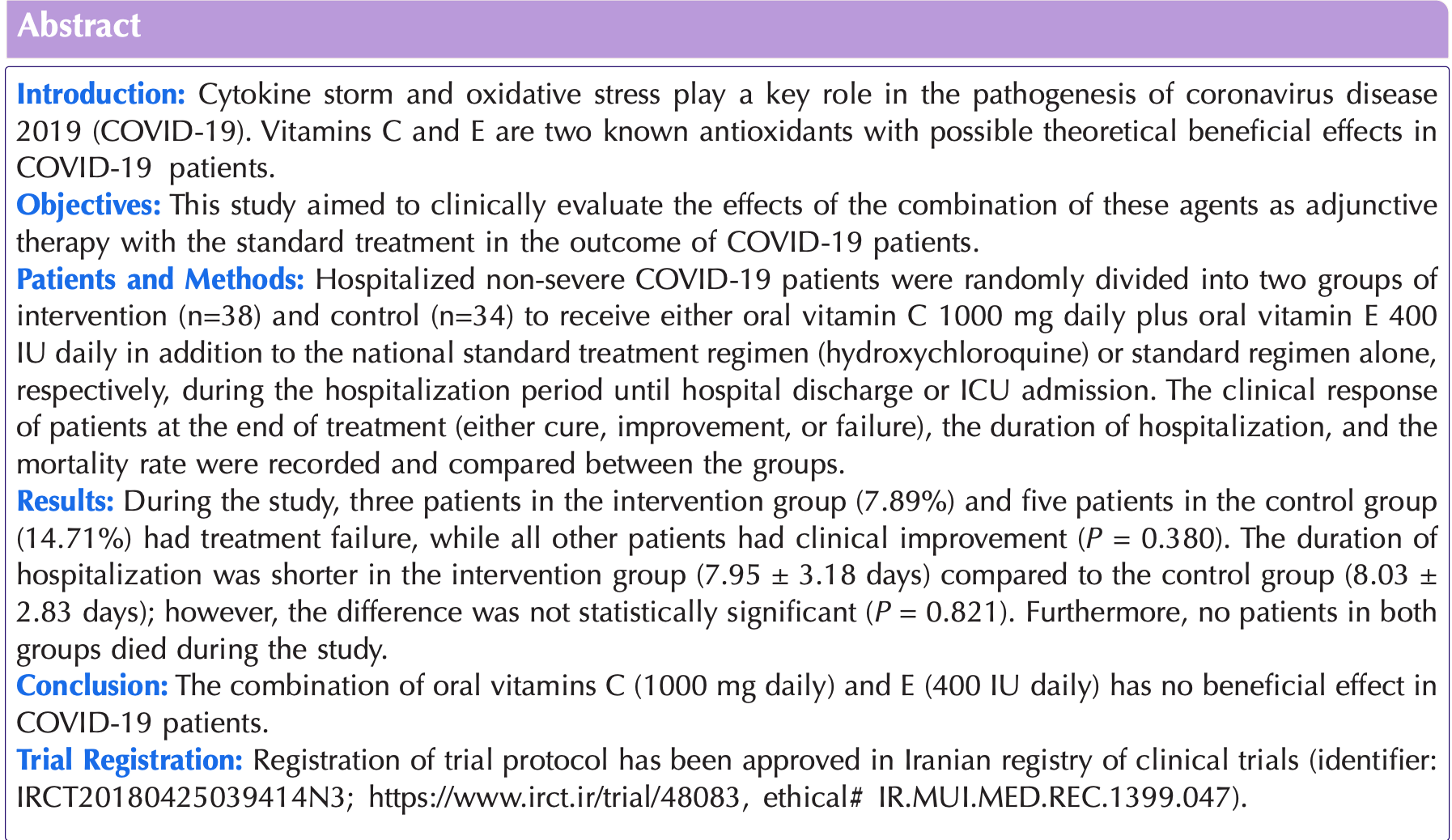
Introduction: Cytokine storm and oxidative stress play a key role in the pathogenesis of coronavirus disease 2019 . Vitamins C and E are two known antioxidants with possible theoretical beneficial effects in COVID-19 patients. Objectives: This study aimed to clinically evaluate the effects of the combination of these agents as adjunctive therapy with the standard treatment in the outcome of COVID-19 patients. Patients and Methods: Hospitalized non-severe COVID-19 patients were randomly divided into two groups of intervention (n=38) and control (n=34) to receive either oral vitamin C 1000 mg daily plus oral vitamin E 400 IU daily in addition to the national standard treatment regimen (hydroxychloroquine) or standard regimen alone, respectively, during the hospitalization period until hospital discharge or ICU admission. The clinical response of patients at the end of treatment (either cure, improvement, or failure), the duration of hospitalization, and the mortality rate were recorded and compared between the groups. Results: During the study, three patients in the intervention group (7.89%) and five patients in the control group (14.71%) had treatment failure, while all other patients had clinical improvement (P = 0.380). The duration of hospitalization was shorter in the intervention group (7.95 ± 3.18 days) compared to the control group (8.03 ± 2.83 days); however, the difference was not statistically significant (P = 0.821). Furthermore, no patients in both groups died during the study.
Conclusion: The combination of oral vitamins C (1000 mg daily) and E (400 IU daily) has no beneficial effect in COVID-19 patients. Trial Registration: Registration of trial protocol has been approved in Iranian registry of clinical trials (identifier: IRCT20180425039414N3; https://www.irct.ir/trial/48083, ethical# IR.MUI.MED.REC.1399.047).
Authors' contribution AH, RS and ARM were the principal investigators of the study. AH and ARM were included in preparing the concept and design. AR and MA revised the manuscript and evaluated the intellectual contents. MJT, SM and KD gathered data. All authors have read and approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
Conflicts of interest The authors declare that they have no competing interests.
Ethical issues The research followed the tenets of the Declaration of Helsinki. The study was registered at the Iranian Registry of Clinical Trials (identifier: IRCT20180425039414N3; https://www.irct. ir/trial/48083). The ethics committee of Isfahan University of Medical Science (IUMS) approved this study with the record number of IR.MUI.MED.REC.1399.047. Written informed consent was obtained from all included patients. Moreover, ethical issues (including plagiarism, data fabrication, double publication) have been completely observed by the authors.
References
Allen, Kelly, Basdeo, Kinsella, Mulready et al., A pilot study of the immunological effects of high-dose vitamin D in healthy volunteers, Mult Scler, doi:10.1177/1352458512442992
Bertrand, Pincemail, Hanique, Denis, Leenaerts et al., Differences in tocopherol-lipid ratios in ARDS and non-ARDS patients, Intensive Care Med, doi:10.1007/BF00295983
Carr, A new clinical trial to test high-dose vitamin C in patients with COVID-19, Crit Care, doi:10.1186/s13054-020-02851-4
Carr, Maggini, Vitamin C and Immune Function, Nutrients, doi:10.3390/nu9111211
Carr, Rosengrave, Bayer, Chambers, Mehrtens et al., Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes, Crit Care, doi:10.1186/s13054-017-1891-y
Cheng, Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)?, Med Drug Discov, doi:10.1016/j.medidd.2020.100028
Delgado-Roche, Mesta, Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection, doi:10.1016/j.arcmed.2020.04.019
Ferreira, Polonini, Dijkers, Postulated Adjuvant Therapeutic Strategies for COVID-19, J Pers Med, doi:10.3390/jpm10030080
Feyaerts, Luyten, Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19, Nutrition, doi:10.1016/j.nut.2020.110948
Goddek, Vitamin D3 and K2 and their potential contribution to reducing the COVID-19 mortality rate, Int J Infect Dis, doi:10.1016/j.ijid.2020.07.080
Hemilä, Chalker, Vitamin C as a Possible Therapy for COVID-19, Infect Chemother, doi:10.3947/ic.2020.52.2.222
Hemilä, Douglas, Vitamin C and acute respiratory infections, The International Journal of Tuberculosis and Lung Disease
Hiedra, Lo, Elbashabsheh, Gul, Wright et al., The use of IV vitamin C for patients with COVID-19: a case series, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2020.1794819
Junejo, Lateef, Eme, Immune System Enriching Micronutrients: Essential Ingredient for Wellbeing in COVID-19, Life Sc, doi:10.37185/LnS.1.1.173
Kang, Vitamin intervention for cytokine storm in the patients with coronavirus disease 2019, Med Comm, doi:10.1002/mco2.7
Lee, Han, The Role of Vitamin E in Immunity, Nutrients, doi:10.3390/nu10111614
Li, Geng, Peng, Meng, Lu, Molecular immune pathogenesis and diagnosis of COVID-19, J Pharm Anal, doi:10.1016/j.jpha.2020.03.001
Mao, Wang, Hu, Chen, He, Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China, JAMA Neurol, doi:10.1001/jamaneurol.2020.1127
Miller, Pastor-Barriuso, Dalal, Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality, Ann Intern Med, doi:10.7326/0003-4819-142-1-200501040-00110
Tian, Rong, Nian, He, Gastrointestinal features in COVID-19 and the possibility of faecal transmission, Aliment Pharmacol Ther, doi:10.1111/apt.15731
Wang, Du, Zhu, Cao, An et al., Comorbidities and multi-organ injuries in the treatment of COVID-19, Lancet, doi:10.1016/S0140-6736(20)30558-4
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Young, Woodside, Antioxidants in health and disease, J Clin Pathol, doi:10.1136/jcp.54.3.176
Zhou, Yang, Wang, Hu, Zhang et al., Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin, BioRxiv, doi:10.1101/2020.01.22.914952