WHO Solidarity Trial Plus: An International Randomised Trial of Additional Treatments for COVID-19 in Hospitalised Patients Who Are All Receiving the Local Standard of Care in Nepal
et al., NCT05273242, NCT05273242, Dec 2022
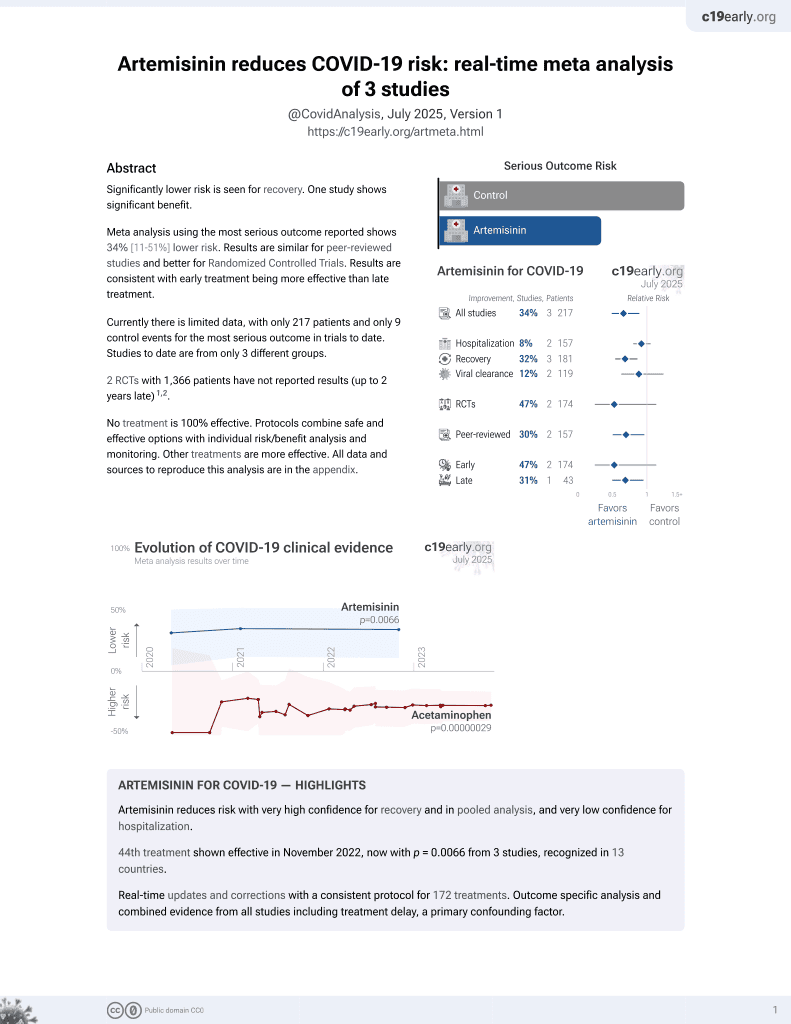
46th treatment shown to reduce risk in
November 2022, now with p = 0.0066 from 3 studies, recognized in 13 countries.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Estimated 400 patient artemisinin late treatment RCT with results not reported over 3 years after estimated completion.
1.
Fowler et al., A Multi-centre, Adaptive, Randomized, Open-label, Controlled Clinical Trial of the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalized Patients (CATCO: Canadian Treatments for COVID-19), in Conjunction With the Public Health Emergency SOLIDARITY Trial (World Health Organization), NCT04330690, clinicaltrials.gov/study/NCT04330690.
Gyanwali et al., 1 Dec 2022, Randomized Controlled Trial, Nepal, trial NCT05273242 (history).
Contact: prgyawali654@gmail.com, clinicaltrialnepal@gmail.com, bideshbista@hotmail.com, pradptiwari@gmail.com, yubarajsharma@pahs.edu.np.