Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(22)00298-3, ACT inpatient, NCT04324463, Oct 2022
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
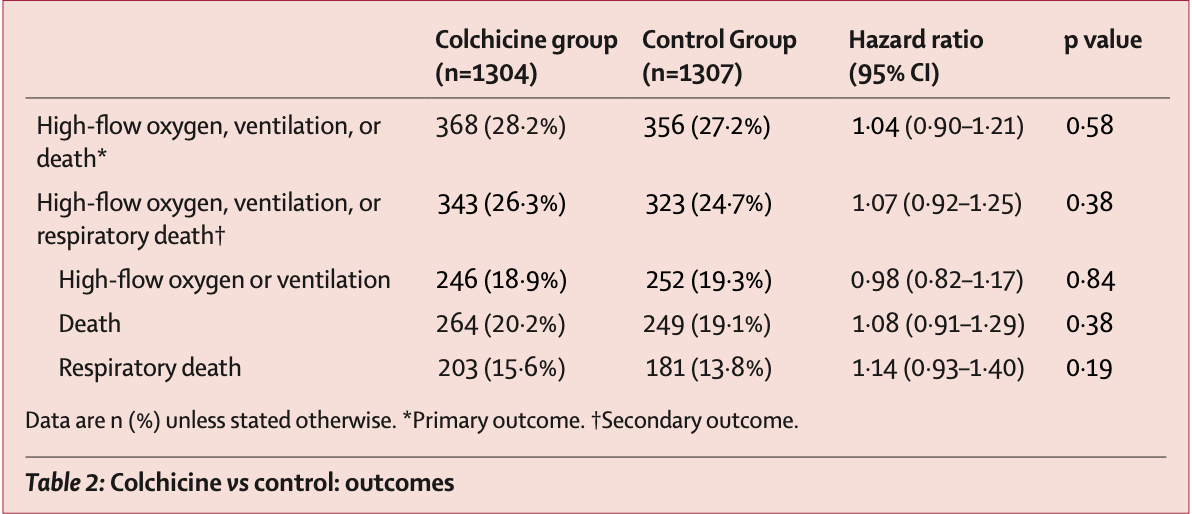
RCT very late stage (baseline SpO2 80%) patients, showing no significant differences with colchicine treatment.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, oxygen saturation <90% at baseline.
Study covers aspirin and colchicine.
|
risk of death, 8.0% higher, HR 1.08, p = 0.38, treatment 264 of 1,304 (20.2%), control 249 of 1,307 (19.1%).
|
|
risk of progression, 4.0% higher, HR 1.04, p = 0.58, treatment 368 of 1,304 (28.2%), control 356 of 1,307 (27.2%), high-flow oxygen, ventilation, or death.
|
|
risk of progression, 2.0% lower, HR 0.98, p = 0.84, treatment 246 of 1,304 (18.9%), control 252 of 1,307 (19.3%), NNT 241, high-flow oxygen or ventilation.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Eikelboom et al., 10 Oct 2022, Randomized Controlled Trial, multiple countries, peer-reviewed, mean age 56.0, 29 authors, study period 2 October, 2020 - 10 February, 2022, average treatment delay 7.0 days, dosage 1.8mg day 1, 1.2mg days 2-28, trial NCT04324463 (history) (ACT inpatient).
Contact: eikelbj@mcmaster.ca.
Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(22)00298-3
Background COVID-19 disease is accompanied by a dysregulated immune response and hypercoagulability. The Anti-Coronavirus Therapies (ACT) inpatient trial aimed to evaluate anti-inflammatory therapy with colchicine and antithrombotic therapy with the combination of rivaroxaban and aspirin for prevention of disease progression in patients hospitalised with COVID-19.
Methods The ACT inpatient, open-label, 2 × 2 factorial, randomised, controlled trial was done at 62 clinical centres in 11 countries. Patients aged at least 18 years with symptomatic, laboratory confirmed COVID-19 who were within 72 h of hospitalisation or worsening clinically if already hospitalised were randomly assigned (1:1) to receive colchicine 1•2 mg followed by 0•6 mg 2 h later and then 0•6 mg twice daily for 28 days versus usual care; and in a second (1:1) randomisation, to the combination of rivaroxaban 2•5 mg twice daily plus aspirin 100 mg once daily for 28 days versus usual care. Investigators and patients were not masked to treatment allocation. The primary outcome, assessed at 45 days in the intention-to-treat population, for the colchicine randomisation was the composite of the need for high-flow oxygen, mechanical ventilation, or death; and for the rivaroxaban plus aspirin randomisation was the composite of major thrombosis (myocardial infarction, stroke, acute limb ischaemia, or pulmonary embolism), the need for high-flow oxygen, mechanical ventilation, or death. The trial is registered at www.clinicaltrials.gov, NCT04324463 and is ongoing. Findings Between Oct 2, 2020, and Feb 10, 2022, at 62 sites in 11 countries, 2749 patients were randomly assigned to colchicine or control and the combination of rivaroxaban and aspirin or to the control. 2611 patients were included in the analysis of colchicine (n=1304) versus control (n=1307); 2119 patients were included in the analysis of rivaroxaban and aspirin (n=1063) versus control (n=1056). Follow-up was more than 98% complete. Overall, 368 (28•2%) of 1304 patients allocated to colchicine and 356 (27•2%) of 1307 allocated to control had a primary outcome (hazard ratio [HR] 1•04, 95% CI 0•90-1•21, p=0•58); and 281 (26•4%) of 1063 patients allocated to the combination of rivaroxaban and aspirin and 300 (28•4%) of 1056 allocated to control had a primary outcome (HR 0•92, 95% CI 0•78-1•09, p=0•32). Results were consistent in subgroups defined by vaccination status, disease severity at baseline, and timing of randomisation in relation to onset of symptoms. There was no increase in the number of patients who had at least one serious adverse event for colchicine versus control groups (87 [6•7%] of 1304 vs 90 [6•9%] of 1307) or with rivaroxaban and aspirin versus control groups (85 [8•0%] vs 91 [8•6%] ). Among patients assigned to colchicine, 8 (0•61%) had adverse events that led to discontinuation of study drug, mostly gastrointestinal in nature. 17 (1•6%) patients assigned to the combination of rivaroxaban and aspirin had..
Declaration of interests JWE reports grant or in-kind support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Pfizer, Janssen, Sanofi-Aventis and honoraria from Astra-Zeneca, Bayer, Boehringer-Ingelheim, Bristol-Myer-Squibb, Daiichi-Sankyo, Eli-Lilly, Glaxo-Smith-Kline, Merck, Pfizer, Janssen, Sanofi-Aventis, Servier. SSJ reports grant support from Boston Scientific, honoraria from Medtronic, Penumbra. EPB-C reports grant support from Bayer, Roche, BMS-Pfizer. RPW reports grant support from Bayer, Roche, BMS-Pfizer, grant and honorarium from Boehringer-Ingelheim, and consultancy fees from Atricure and Phasebio. MLD reports grant support from the Population Health Research Institute (PHRI) to manage the ACT study in Argentina. RD reports grant support from PHRI to manage the ACT study in Argentina. AA reports institutional grant support from Bayer and EMS, and lecture fees from Bayer and Sanofi-Aventis. SW reports grant support from NIH, honoraria from Pfizer, and safety monitoring committee of an AIDS Clinical Trial Group. RDL reports institutional grant support from Bristol Myers Squibb, Glaxo Smith Kline, Medtronic, Pfizer, and Sanofi, consulting fees from Bristol Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, Sanofi, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck, and Portola, honoraria from Pfizer and meeting travel support from IQVIA. OB reports grant support from Astra Zeneca, Bayer, Amgen, Novartis, Servier, Pfizer. SSA..
References
Andrews, Stowe, Kirsebom, Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant, N Engl J Med
Baigent, Blackwell, Collins, Aspirin in the primary and secondary prevention of vascular disease: collaborative metaanalysis of individual participant data from randomised trials, Lancet
Bartoletti, Azap, Barac, ESCMID COVID-19 living guidelines: drug treatment and clinical management, Clin Microbiol Infect
Berlin, Gulick, Martinez, Severe Covid-19, N Engl J Med
Bollyky, Nuzzo, Huhn, Kiernan, Pond, Global vaccination must be swifter, Nature
Bonaventura, Vecchié, Dagna, Tangianu, Abbate et al., Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation, Inflamm Res
Bradbury, Lawler, Stanworth, Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial, JAMA
Diaz, Orlandini, Castellana, Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial, JAMA Netw Open
Eikelboom, Connolly, Bosch, Rivaroxaban with or without aspirin in stable cardiovascular disease, N Engl J Med
Eikelboom, Jolly, Belley-Cote, Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00299-5
Eikelboom, Rangarajan, Jolly, The anti-coronavirus therapies (ACT) trials: design, baseline characteristics, and challenges, CJC Open
Goligher, Bradbury, Mcverry, Therapeutic anticoagulation with heparin in critically ill patients with Covid-19, N Engl J Med
Group, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Group, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet Respir Med
Hoenigl, Seidel, Carvalho, The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries, Lancet Microbe
Lawler, Goligher, Berger, Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19, N Engl J Med
Leentjens, Van Haaps, Wessels, Schutgens, Middeldorp, COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year, Lancet Haematol
Lopes, De, Silva, Furtado, Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial, Lancet
Maiese, Manetti, Russa, Autopsy findings in COVID-19-related deaths: a literature review, Forensic Sci Med Pathol
Oliynyk, Barg, Slifirczyk, Comparison of the effect of unfractionated heparin and enoxaparin sodium at different doses on the course of COVID-19-associated coagulopathy, Life
Pavia, Pasteur, vaccines, and the refusal to become fully vaccinated in the midst of the COVID-19 pandemic, Front Public Health
Perepu, Chambers, Wahab, Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial, J Thromb Haemost
Sadeghipour, Talasaz, Rashidi, Effect of intermediatedose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial, JAMA
Sanghavi, Bansal, Kaur, Impact of colchicine on mortality and morbidity in COVID-19: a systematic review, Ann Med
Sholzberg, Tang, Rahhal, Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial, BMJ
Spyropoulos, Goldin, Giannis, Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediatedose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial, JAMA Intern Med
Sterne, Diaz, Villar, Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials, Trials
Tardif, Bouabdallaoui, Allier, (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebocontrolled, multicentre trial, Lancet Respir Med
Wang, Chen, Yang, Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19, J Med Virol
Wills, Nair, Patel, Efficacy and safety of intensified versus standard prophylactic anticoagulation therapy in patients with Covid-19: a systematic review and meta-analysis, Open Forum Infect Dis
DOI record:
{
"DOI": "10.1016/s2213-2600(22)00298-3",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(22)00298-3",
"alternative-id": [
"S2213260022002983"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(22)00298-3"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(22)00368-X"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Eikelboom",
"given": "John W",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jolly",
"given": "Sanjit S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belley-Cote",
"given": "Emilie P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Whitlock",
"given": "Richard P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rangarajan",
"given": "Sumathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Lizhen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heenan",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bangdiwala",
"given": "Shrikant I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Luz Diaz",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diaz",
"given": "Rafael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yusufali",
"given": "Afzalhussein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar Sharma",
"given": "Sanjib",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarhuni",
"given": "Wadea M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hassany",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avezum",
"given": "Alvaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harper",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wasserman",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almas",
"given": "Aysha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drapkina",
"given": "Oxana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Felix",
"given": "Camilo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopes",
"given": "Renato D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berwanger",
"given": "Otavio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Jaramillo",
"given": "Patricio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anand",
"given": "Sonia S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bosch",
"given": "Jackie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhri",
"given": "Shurjeel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farkouh",
"given": "Michael E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loeb",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yusuf",
"given": "Salim",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T22:31:54Z",
"timestamp": 1665441114000
},
"deposited": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T22:32:24Z",
"timestamp": 1665441144000
},
"indexed": {
"date-parts": [
[
2022,
10,
10
]
],
"date-time": "2022-10-10T23:12:03Z",
"timestamp": 1665443523343
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
1
]
],
"date-time": "2022-10-01T00:00:00Z",
"timestamp": 1664582400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002983?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002983?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMcp2009249",
"article-title": "Mild or moderate Covid-19",
"author": "Gandhi",
"doi-asserted-by": "crossref",
"first-page": "1757",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00298-3_bib1",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009575",
"article-title": "Severe Covid-19",
"author": "Berlin",
"doi-asserted-by": "crossref",
"first-page": "2451",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00298-3_bib2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2021.11.007",
"article-title": "ESCMID COVID-19 living guidelines: drug treatment and clinical management",
"author": "Bartoletti",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/S2213-2600(22)00298-3_bib3",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S2666-5247(21)00237-8",
"article-title": "The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries",
"author": "Hoenigl",
"doi-asserted-by": "crossref",
"first-page": "e543",
"journal-title": "Lancet Microbe",
"key": "10.1016/S2213-2600(22)00298-3_bib4",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/d41586-022-00809-w",
"article-title": "Global vaccination must be swifter",
"author": "Bollyky",
"doi-asserted-by": "crossref",
"first-page": "788",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(22)00298-3_bib5",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.3389/fpubh.2022.815816",
"article-title": "Pasteur, vaccines, and the refusal to become fully vaccinated in the midst of the COVID-19 pandemic",
"author": "Pavia",
"doi-asserted-by": "crossref",
"journal-title": "Front Public Health",
"key": "10.1016/S2213-2600(22)00298-3_bib6",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2119451",
"article-title": "Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant",
"author": "Andrews",
"doi-asserted-by": "crossref",
"first-page": "1532",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00298-3_bib7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1007/s00011-022-01540-y",
"article-title": "Colchicine for COVID-19: targeting NLRP3 inflammasome to blunt hyperinflammation",
"author": "Bonaventura",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "Inflamm Res",
"key": "10.1016/S2213-2600(22)00298-3_bib8",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1080/07853890.2021.1993327",
"article-title": "Impact of colchicine on mortality and morbidity in COVID-19: a systematic review",
"author": "Sanghavi",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "Ann Med",
"key": "10.1016/S2213-2600(22)00298-3_bib9",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofac285",
"doi-asserted-by": "crossref",
"key": "10.1016/S2213-2600(22)00298-3_bib10",
"unstructured": "Wills NK, Nair N, Patel K, et al. Efficacy and safety of intensified versus standard prophylactic anticoagulation therapy in patients with Covid-19: a systematic review and meta-analysis. Open Forum Infect Dis; 9: ofac285."
},
{
"DOI": "10.1056/NEJMoa1709118",
"article-title": "Rivaroxaban with or without aspirin in stable cardiovascular disease",
"author": "Eikelboom",
"doi-asserted-by": "crossref",
"first-page": "1319",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00298-3_bib11",
"volume": "377",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(09)60503-1",
"article-title": "Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials",
"author": "Baigent",
"doi-asserted-by": "crossref",
"first-page": "1849",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00298-3_bib12",
"volume": "373",
"year": "2009"
},
{
"DOI": "10.1016/j.cjco.2022.02.010",
"article-title": "The anti-coronavirus therapies (ACT) trials: design, baseline characteristics, and challenges",
"author": "Eikelboom",
"doi-asserted-by": "crossref",
"first-page": "568",
"journal-title": "CJC Open",
"key": "10.1016/S2213-2600(22)00298-3_bib13",
"volume": "4",
"year": "2022"
},
{
"article-title": "Colchicine and aspirin in community patients with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial",
"author": "Eikelboom",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00298-3_bib14",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)01203-4",
"article-title": "Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial",
"author": "Lopes",
"doi-asserted-by": "crossref",
"first-page": "2253",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00298-3_bib15",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.6203",
"article-title": "Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial",
"author": "Spyropoulos",
"doi-asserted-by": "crossref",
"first-page": "1612",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S2213-2600(22)00298-3_bib16",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.4152",
"author": "Sadeghipour",
"doi-asserted-by": "crossref",
"first-page": "1620",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00298-3_bib17",
"volume": "325",
"year": "2021"
},
{
"article-title": "Comparison of the effect of unfractionated heparin and enoxaparin sodium at different doses on the course of COVID-19-associated coagulopathy",
"author": "Oliynyk",
"journal-title": "Life (Basel)",
"key": "10.1016/S2213-2600(22)00298-3_bib18",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1111/jth.15450",
"article-title": "Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial",
"author": "Perepu",
"doi-asserted-by": "crossref",
"first-page": "2225",
"journal-title": "J Thromb Haemost",
"key": "10.1016/S2213-2600(22)00298-3_bib19",
"volume": "19",
"year": "2021"
},
{
"article-title": "Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial",
"author": "Sholzberg",
"journal-title": "BMJ",
"key": "10.1016/S2213-2600(22)00298-3_bib20",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2105911",
"article-title": "Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19",
"author": "Lawler",
"doi-asserted-by": "crossref",
"first-page": "790",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00298-3_bib21",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103417",
"article-title": "Therapeutic anticoagulation with heparin in critically ill patients with Covid-19",
"author": "Goligher",
"doi-asserted-by": "crossref",
"first-page": "777",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00298-3_bib22",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04641-3",
"article-title": "Corticosteroid therapy for critically ill patients with COVID-19: A structured summary of a study protocol for a prospective meta-analysis of randomized trials",
"author": "Sterne",
"doi-asserted-by": "crossref",
"first-page": "734",
"journal-title": "Trials",
"key": "10.1016/S2213-2600(22)00298-3_bib24",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.11330",
"article-title": "Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "499",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00298-3_bib25",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "1419",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00298-3_bib26",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.41328",
"article-title": "Effect of colchicine vs usual care alone on intubation and 28-day mortality in patients hospitalized with COVID-19: a randomized clinical trial",
"author": "Diaz",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/S2213-2600(22)00298-3_bib27",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif",
"doi-asserted-by": "crossref",
"first-page": "924",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(22)00298-3_bib28",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2352-3026(21)00105-8",
"article-title": "COVID-19-associated coagulopathy and antithrombotic agents-lessons after 1 year",
"author": "Leentjens",
"doi-asserted-by": "crossref",
"first-page": "e524",
"journal-title": "Lancet Haematol",
"key": "10.1016/S2213-2600(22)00298-3_bib29",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1007/s12024-020-00310-8",
"article-title": "Autopsy findings in COVID-19-related deaths: a literature review",
"author": "Maiese",
"doi-asserted-by": "crossref",
"first-page": "279",
"journal-title": "Forensic Sci Med Pathol",
"key": "10.1016/S2213-2600(22)00298-3_bib30",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1001/jama.2022.2910",
"article-title": "Effect of antiplatelet therapy on survival and organ support-free days in critically ill patients with COVID-19: a randomized clinical trial",
"author": "Bradbury",
"doi-asserted-by": "crossref",
"first-page": "1247",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00298-3_bib31",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)01825-0",
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00298-3_bib32",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1002/jmv.26771",
"article-title": "Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "2908",
"journal-title": "J Med Virol",
"key": "10.1016/S2213-2600(22)00298-3_bib23",
"volume": "93",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260022002983"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Colchicine and the combination of rivaroxaban and aspirin in patients hospitalised with COVID-19 (ACT): an open-label, factorial, randomised, controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}
