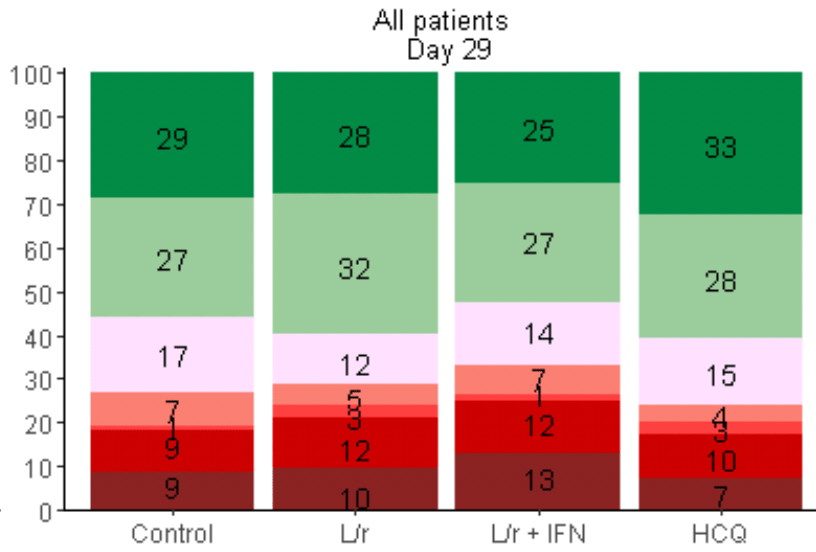
An open-label randomized, controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-beta-1a and hydroxychloroquine in hospitalized patients with COVID-19 - Final results from the DisCoVeRy trial
et al., medRxiv, doi:10.1101/2022.02.16.22271064, Oct 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Early terminated very late stage (95% on oxygen at baseline) DISCOVERY trial. 4% more patients were on ventilation at baseline in the HCQ group. This preprint presents more recent results than the earlier journal article.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, >50% on oxygen/ventilation at baseline.
Study covers lopinavir/ritonavir and HCQ.
|
risk of death, 15.3% higher, RR 1.15, p = 0.70, treatment 11 of 150 (7.3%), control 13 of 149 (8.7%), adjusted per study, odds ratio converted to relative risk, day 90.
|
|
risk of death, 10.1% lower, RR 0.90, p = 0.75, treatment 15 of 150 (10.0%), control 13 of 149 (8.7%), adjusted per study, odds ratio converted to relative risk, day 28.
|
|
risk of no viral clearance, 23.8% lower, RR 0.76, p = 0.68, treatment 4 of 83 (4.8%), control 5 of 81 (6.2%), NNT 74, odds ratio converted to relative risk, Table S2, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ader et al., 6 Oct 2020, Randomized Controlled Trial, multiple countries, preprint, baseline oxygen required 95.4%, 59 authors, study period 22 March, 2020 - 29 June, 2020, average treatment delay 9.0 days.
Contact: florence.ader@chu-lyon.fr.
An open-label randomized, controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19 – Final results from the DisCoVeRy trial
doi:10.1101/2022.02.16.22271064
An open-label randomized, controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19 -Final results from the DisCoVeRy trial.
Authors contribution Writing -Original Draft: FA, CB; Writing -Review & Editing: NPS, JP, MBD, DB, ADi, MH, MPL, GP, DC, YY, FM; Conceptualization: FA, NPS, JP, MBD, GP, BL, DC, YY, FM; Investigation: FA, NPS, JP, MBD, Adi, NM, FXL, FR, FG, AK, SJ, JR, SN, FD, RCJ, KB, JCN, VT, AC, CDu, JC, SL, JM, RG, BM, EF, VP, SG, OL, KL, JPL, AM, GMB, LB, ÉBN, AGB, OE, LP, FW, JCR, JR, TS, MH, CA, MPL, GP; Methodology: FA, NPS, JP, MBD, DC, CB, FM; Data curation: ADi, ADe, NM, ADu, TA; Formal Analysis: DB, ADu, DC, CB, FM Project Administration: FA, CD, FM; Funding Acquisition: FA, CDe, JS, DC, YY, FM.
Declaration of interests F.R. reports personal fees from Gilead Sciences, personal fees from MSD, personal fees from Pfizer, personal fees from TheraTechnologies, personal fees from ViiV Healthcare, outside the submitted work. F.G. reports grants from BioMerieux, personal fees and non-financial support from Gilead, non-financial support from Corevio, outside the submitted work. G.P. reports grants and personal fees from Gilead Sciences, grants and personal fees from Merck, grants and personal fees from ViiV Healthcare, grants and personal fees from TheraTechnologies, outside the submitted work. K.L. reports personal fees and non-financial support from Gilead, personal fees and non-financial support from Janssen, personal fees and non-financial support from MSD, personal fees and non-financial support from ViiV Healthcare, personal fees and non-financial support from Abbvie, during..
References
Ader, Bouscambert-Duchamp, Hites, Peiffer-Smadja, Poissy et al., Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial, The Lancet Infectious Diseases
Ader, Discovery, Management, Protocol for the DisCoVeRy trial: multicentre, adaptive, randomised trial of the safety and efficacy of treatments for COVID-19 in hospitalised adults, BMJ open
Ader, Peiffer-Smadja, Poissy, Bouscambert-Duchamp, Belhadi et al., An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-β-1a and hydroxychloroquine in hospitalized patients with COVID-19, Clin Microbiol Infect
Ap-Hp, Bichat-Claude, Bernard, Service de réanimation médicale et infectieuse
Ap-Hp, Hôpital, Mondor, Service d'immunologie et maladies infectieuses
Ap-Hp, None
Aphp, Saint-Antoine, Service de maladies infectieuses et tropicales, F-75012
Arabi, Alothman, Balkhy, Al-Dawood, Aljohani et al., Treatment of Middle East Respiratory Syndrome with a combination of lopinavirritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial, Trials
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N Engl J Med
Cavalcanti, Zampieri, Rosa, Azevedo, Veiga et al., Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19, The New England journal of medicine
Chhonker, Sleightholm, Li, Oupický, Murry, Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC-ESI-MS/MS: An application for pharmacokinetic studies, Journal of Chromatography B. janv
Choy, Wong, Kaewpreedee, Sia, Chen et al., Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral research
Chu De Dijon, Département de maladies infectieuses, F-21000
Chu De Montpellier, None
Chu De Saint-Etienne, Service d'Infectiologie, F-42055
Chu, Cheng, Hung, Wong, Chan et al., Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings, Thorax
Clementi, Ferrarese, Criscuolo, Diotti, Castelli et al., Interferon-beta-1a Inhibition of Severe Acute Respiratory Syndrome-Coronavirus 2 In Vitro When Administered After Virus Infection, The Journal of infectious diseases
Croxtall, Perry, Lopinavir/Ritonavir: a review of its use in the management of HIV-1 infection, Drugs
De Lyon, Département de soins intensifs, F-69000
De Wilde, Jochmans, Posthuma, Zevenhoven-Dobbe, Van Nieuwkoop et al., Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrobial agents and chemotherapy
Etievant, Bal, Escuret, Brengel-Pesce, Bouscambert et al., Performance Assessment of SARS-CoV-2 PCR Assays Developed by WHO Referral Laboratories, J Clin Med
Horby, Mafham, Bell, Linsell, Staplin et al., Lopinavirritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, The Lancet
Jung, Rezk, Bridges, Corbett, Kashuba, Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry, Biomed Chromatogr
Kaletra, Summary of Product Characteristics
Le, Peiffer-Smadja, Guedj, Neant, Mentre et al., Rationale of a loading dose initiation for hydroxychloroquine treatment in COVID-19 infection in the DisCoVeRy trial. The Journal of antimicrobial chemotherapy
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell discovery
Lokugamage, Hage, De Vries, Valero-Jimenez, Schindewolf et al., Type I Interferon Susceptibility Distinguishes SARS-CoV-2 from SARS-CoV, J Virol
Maisonnasse, Guedj, Contreras, Behillil, Solas et al., Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates, Nature. sept
Marzolini, Stader, Stoeckle, Franzeck, Egli et al., Effect of Systemic Inflammatory Response to SARS-CoV-2 on Lopinavir and Hydroxychloroquine Plasma Concentrations, Antimicrob Agents Chemother
Ofotokun, Lennox, Eaton, Ritchie, Easley et al., Immune activation mediated change in alpha-1-acid glycoprotein: impact on total and free lopinavir plasma exposure, J Clin Pharmacol. nov
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Recovery Collaborative Group, Horby, Mafham, Linsell, Bell et al., Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med
Sallard, Lescure, Yazdanpanah, Mentre, Peiffer-Smadja, Type 1 interferons as a potential treatment against COVID-19, Antiviral research
Thakur, Tan, Chan, Physiologically-Based Pharmacokinetic Modeling to Predict the Clinical Efficacy of the Coadministration of Lopinavir and Ritonavir against SARS-CoV-2, Clinical pharmacology and therapeutics
Warren, Jordan, Lo, Ray, Mackman et al., Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature
Yao, Ye, Zhang, Cui, Huang et al., Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
Zhu, Zhang, Li, Yang, Song, A Novel Coronavirus from Patients with Pneumonia in China, 2019, The New England journal of medicine
DOI record:
{
"DOI": "10.1101/2022.02.16.22271064",
"URL": "http://dx.doi.org/10.1101/2022.02.16.22271064",
"abstract": "<jats:p>Objectives\nWe evaluated the clinical, virological and safety outcomes of lopinavir/ritonavir, lopinavir/ritonavir-interferon (IFN)-beta-1a, hydroxychloroquine or remdesivir in comparison to standard of care (control) in COVID-19 inpatients requiring oxygen and/or ventilatory support. While preliminary results were previously published, we present here the final results, following completion of the data monitoring.\nMethods\nWe conducted a phase 3 multi-centre open-label, randomized 1:1:1:1:1, adaptive, controlled trial (DisCoVeRy), add-on trial to Solidarity (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04315948\">NCT04315948</jats:ext-link>, EudraCT2020-000936-23). The primary outcome was the clinical status at day 15, measured by the WHO 7-point ordinal scale. Secondary outcomes included SARS-CoV-2 quantification in respiratory specimens, pharmacokinetic and safety analyses. We report the results for the lopinavir/ritonavir-containing arms and for the hydroxychloroquine arm, which were stopped prematurely.\nResults\nThe intention-to-treat population included 593 participants (lopinavir/ritonavir, n=147; lopinavir/ritonavir-IFN-beta-1a, n=147; hydroxychloroquine, n=150; control, n=149), among whom 421 (71.0%) were male, the median age was 64 years (IQR, 54-71) and 214 (36.1%) had a severe disease. The day 15 clinical status was not improved with investigational treatments: lopinavir/ritonavir versus control, adjusted odds ratio (aOR) 0.82, (95% confidence interval [CI] 0.54-1.25, P=0.36); lopinavir/ritonavir-IFN-beta-1a versus control, aOR 0.69 (95%CI 0.45-1.05, P=0.08); hydroxychloroquine versus control, aOR 0.94 (95%CI 0.62-1.41, P=0.76). No significant effect of investigational treatment was observed on SARS-CoV-2 clearance. Trough plasma concentrations of lopinavir and ritonavir were higher than those expected, while those of hydroxychloroquine were those expected with the dosing regimen. The occurrence of Serious Adverse Events was significantly higher in participants allocated to the lopinavir/ritonavir-containing arms. \nConclusion\nIn adults hospitalized for COVID-19, lopinavir/ritonavir, lopinavir/ritonavir-IFN-beta-1a and hydroxychloroquine did not improve the clinical status at day 15, nor SARS-CoV-2 clearance in respiratory tract specimens.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
2,
21
]
]
},
"author": [
{
"affiliation": [],
"family": "ADER",
"given": "Florence",
"sequence": "first"
},
{
"affiliation": [],
"family": "PEIFFER-SMADJA",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "POISSY",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BOUSCAMBERT-DUCHAMP",
"given": "Maude",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BELHADI",
"given": "Drifa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DIALLO",
"given": "Alpha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DELMAS",
"given": "Christelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "SAILLARD",
"given": "Juliette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DECHANET",
"given": "Aline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MERCIER",
"given": "Noemie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DUPONT",
"given": "Axelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ALFAIATE",
"given": "Toni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LESCURE",
"given": "Francois-Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "RAFFI",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "GOEHRINGER",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "KIMMOUN",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "JAUREGUIBERRY",
"given": "Stephane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "REIGNIER",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "NSEIR",
"given": "Saad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DANION",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "CLERE-JEHL",
"given": "Raphael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BOUILLER",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "NAVELLOU",
"given": "Jean-Christophe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "TOLSMA",
"given": "Violaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "CABIE",
"given": "Andre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DUBOST",
"given": "Clement",
"sequence": "additional"
},
{
"affiliation": [],
"family": "COURJON",
"given": "Johan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LEROY",
"given": "Sylvie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MOOTIEN",
"given": "Joy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "GACI",
"given": "Rostane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MOURVILLIER",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "FAURE",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "POURCHER",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "GALLIEN",
"given": "Sebastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LAUNAY",
"given": "Odile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LACOMBE",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LANOIX",
"given": "Jean-Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MAKINSON",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MARTIN-BLONDEL",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BOUADMA",
"given": "Lila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BOTELHO-NEVERS",
"given": "elisabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "GAGNEUX-BRUNON",
"given": "Amandine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "EPAULARD",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "PIROTH",
"given": "Lionel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "WALLET",
"given": "Florent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "RICHARD",
"given": "Jean-Christophe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "REUTER",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "STAUB",
"given": "Therese",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LINA",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "NORET",
"given": "Marion",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ANDREJAK",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LE",
"given": "Minh-Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "PEYTAVIN",
"given": "Gilles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "HITES",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "COSTAGLIOLA",
"given": "Dominique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "YAZDANPANAH",
"given": "Yazdan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "BURDET",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MENTRE",
"given": "France",
"sequence": "additional"
},
{
"affiliation": [],
"name": "DisCoVeRy study group",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
21
]
],
"date-time": "2022-02-21T21:00:20Z",
"timestamp": 1645477220000
},
"deposited": {
"date-parts": [
[
2022,
2,
21
]
],
"date-time": "2022-02-21T21:00:20Z",
"timestamp": 1645477220000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
2,
21
]
],
"date-time": "2022-02-21T21:41:45Z",
"timestamp": 1645479705879
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
2,
21
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.02.16.22271064",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
2,
21
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
2,
21
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"An open-label randomized, controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-beta-1a and hydroxychloroquine in hospitalized patients with COVID-19 - Final results from the DisCoVeRy trial"
],
"type": "posted-content"
}
