Impact of Rapidly Deployed COVID-19 Monoclonal Antibody Infusion Clinics on Rate of Hospitalization
et al., Infectious Diseases in Clinical Practice, doi:10.1097/IPC.0000000000001109, Jan 2022
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
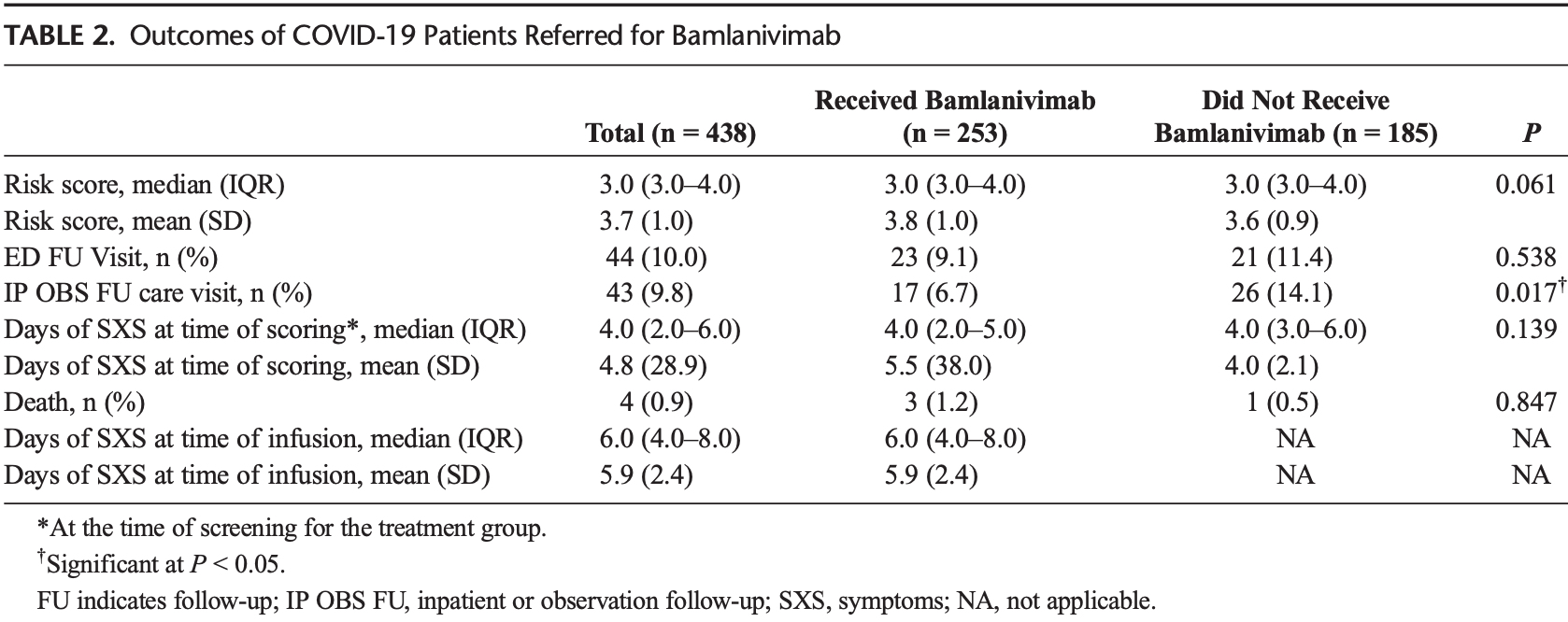
Retrospective 438 patients in the USA, 253 treated with bamlanivimab, showing significantly lower hospitalization with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 119.4% higher, RR 2.19, p = 0.64, treatment 3 of 253 (1.2%), control 1 of 185 (0.5%).
|
|
risk of hospitalization, 52.2% lower, RR 0.48, p = 0.01, treatment 17 of 253 (6.7%), control 26 of 185 (14.1%), NNT 14.
|
|
risk of progression, 19.9% lower, RR 0.80, p = 0.52, treatment 23 of 253 (9.1%), control 21 of 185 (11.4%), NNT 44, ER followup visit.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Delasobera et al., 27 Jan 2022, retrospective, USA, peer-reviewed, 12 authors.
Impact of Rapidly Deployed COVID-19 Monoclonal Antibody Infusion Clinics on Rate of Hospitalization
Background: Providing monoclonal antibody therapy infusions requires challenging logistics, resources, and technology, hindering availability across the United States. Early identification and treatment of COVIDpositive patients can reduce hospitalizations. Objective: This study aimed to describe the referral, selection process, and deployment of outpatient monoclonal infusion clinics, as well as the impact of monoclonal antibody therapy in COVID-positive patients on the rate of emergency department (ED) visits and hospitalization. Methods: This is a retrospective cohort study that used screening of all COVID-positive ambulatory patients using a unique scoring rubric embedded in the emergency medical response for appropriateness for therapy between November 2020 and January 2021. Participants included all outpatients testing positive for COVID-19 were screened, and those eligible were referred for treatment with bamlanivimab. Of the 443 patients referred for treatment, 252 patients were treated with bamlanivimab compared with 191 patients who declined treatment. Patients were treated either in 1 of the 2 outpatient infusion centers (74%) or the ED (26%.) Results: Of 443 patients with positive COVID-19 diagnoses who were eligible for treatment based on a risk assessment rubric, 252 received bamlanivimab. There was a significant reduction in hospitalization of the treatment versus control group (6.7% vs 13.6%, P < 0.05). No significant differences were noted in risk score at screening, ED visits after infusion, days of symptom onset at screening or infusion, or death.
Conclusions and Relevance: The efficacy of monoclonal antibody therapy on reducing hospitalization was demonstrated. Rapid development of screening technology, scheduling and operational logistics, and physical space can overcome the challenges in the current environment.
References
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19, N Engl J Med
Fineberg, Rapid Expert Consultation on Allocating COVID-19 Monoclonal Antibody Therapies and Other Novel Therapeutics, doi:10.17226/26063
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Kumar, Wu, Stosor, Real-world experience of bamlanivimab for COVID-19: a case-control study, Clin Infect Dis, doi:10.1093/cid/ciab305
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19, N Engl J Med
Williamson, Tewarson, Increasing utilization of COVID-19 monoclonal antibodies: considerations and promising practices for states
DOI record:
{
"DOI": "10.1097/ipc.0000000000001109",
"ISSN": [
"1536-9943",
"1056-9103"
],
"URL": "http://dx.doi.org/10.1097/ipc.0000000000001109",
"author": [
{
"affiliation": [],
"family": "Delasobera",
"given": "Bronson E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Saggar",
"given": "Tara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodwin",
"given": "Jennifer N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joy",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henry",
"given": "Kiersten N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levin",
"given": "Bonnie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hager",
"given": "David D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McAlduff",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DeSale",
"given": "Sameer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owens",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wortmann",
"given": "Glenn W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Princy N.",
"sequence": "additional"
}
],
"container-title": [
"Infectious Diseases in Clinical Practice"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
26
]
],
"date-time": "2022-01-26T18:00:08Z",
"timestamp": 1643220008000
},
"deposited": {
"date-parts": [
[
2022,
1,
27
]
],
"date-time": "2022-01-27T09:00:25Z",
"timestamp": 1643274025000
},
"indexed": {
"date-parts": [
[
2022,
1,
28
]
],
"date-time": "2022-01-28T06:56:28Z",
"timestamp": 1643352988200
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1536-9943"
},
{
"type": "print",
"value": "1056-9103"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2022,
1,
27
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/IPC.0000000000001109",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "1-6",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2022,
1,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19",
"doi-asserted-by": "crossref",
"first-page": "229",
"journal-title": "N Engl J Med",
"key": "bib1-20220127",
"volume": "384",
"year": "2021"
},
{
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "bib2-20220127",
"volume": "384",
"year": "2020"
},
{
"article-title": "Real-world experience of bamlanivimab for COVID-19: a case-control study",
"journal-title": "Clin Infect Dis",
"key": "bib5-20220127",
"volume": "ciab305",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"doi-asserted-by": "crossref",
"first-page": "632",
"issue": "7",
"journal-title": "JAMA",
"key": "bib6-20220127",
"volume": "325",
"year": "2021"
}
],
"reference-count": 4,
"references-count": 4,
"relation": {},
"score": 1,
"short-container-title": [
"Infect Dis Clin Pract"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"Impact of Rapidly Deployed COVID-19 Monoclonal Antibody Infusion Clinics on Rate of Hospitalization"
],
"type": "journal-article",
"volume": "30"
}
