Clinical Outcomes of Monoclonal Antibody Therapy During a COVID-19 Outbreak in a Skilled Nursing Facility - Arizona, 2021
et al., Journal of the American Geriatrics Society, doi:10.1111/jgs.17705, Feb 2022
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 75 COVID+ patients in a skilled nursing facility in the USA, 56 treated within a median of 2 days from symptom onset with bamlanivimab, showing significantly lower mortality with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
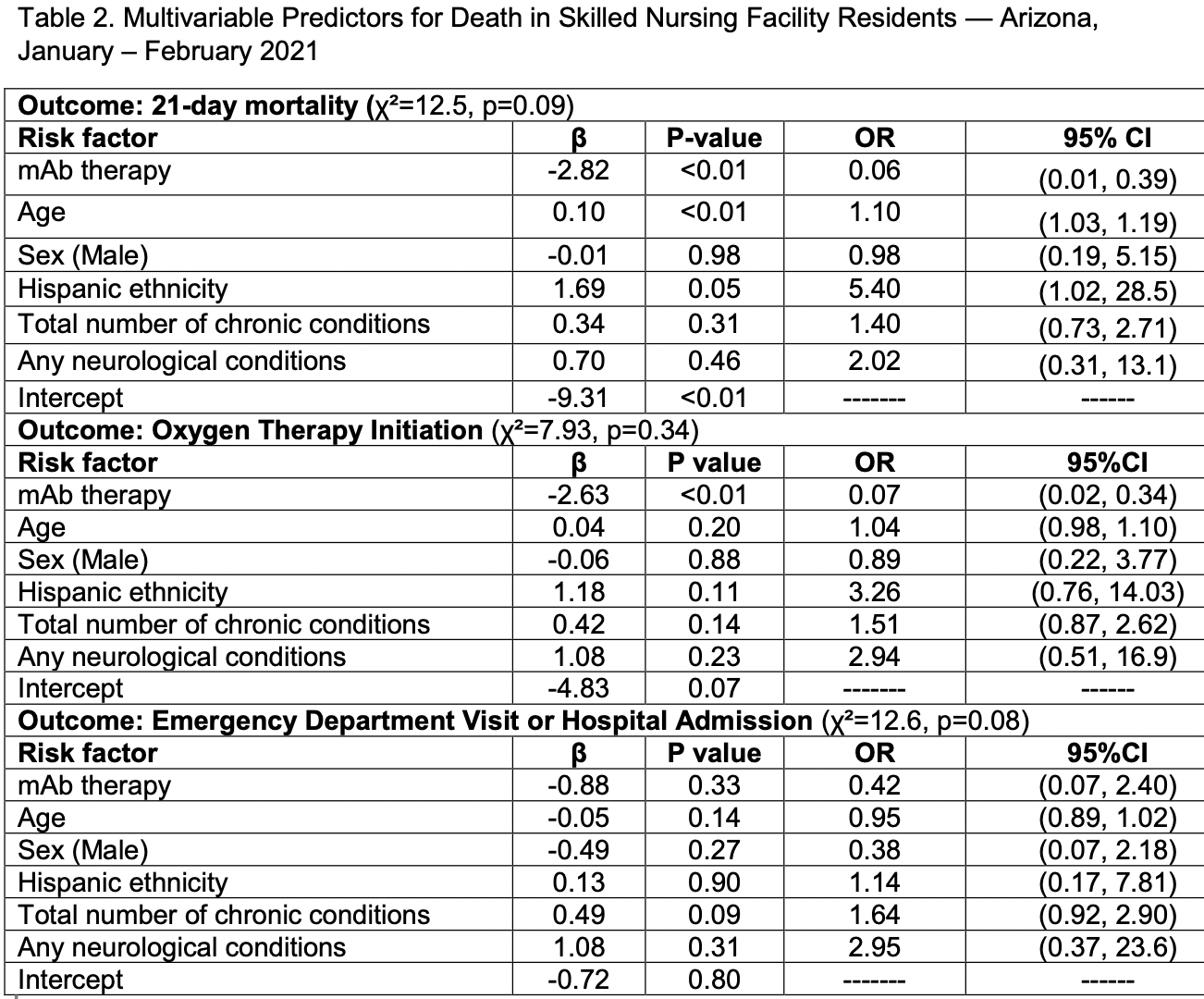
risk of death, 89.2% lower, RR 0.11, p = 0.010, treatment 5 of 56 (8.9%), control 9 of 19 (47.4%), NNT 2.6, adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
risk of progression, 86.3% lower, RR 0.14, p = 0.002, treatment 6 of 56 (10.7%), control 10 of 19 (52.6%), NNT 2.4, adjusted per study, odds ratio converted to relative risk, oxygen therapy, multivariable.
|
|
risk of progression, 53.8% lower, RR 0.46, p = 0.35, treatment 6 of 56 (10.7%), control 3 of 19 (15.8%), adjusted per study, odds ratio converted to relative risk, ER visit or hospitalization, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Dale et al., 9 Feb 2022, retrospective, USA, peer-reviewed, 14 authors, average treatment delay 2.0 days.
Clinical outcomes of monoclonal antibody therapy during a COVID ‐19 outbreak in a skilled nursing facility—Arizona, 2021
Journal of the American Geriatrics Society, doi:10.1111/jgs.17705
Receipt of monoclonal antibody therapy significantly reduced odds of mortality and need for supplemental oxygen in skilled nursing facility residents with mild-to-moderate COVID-19. Use of monoclonal antibody therapy in skilled nursing facilities requires close partnership with local health departments and healthcare entities
Why does this paper matter? This paper describes the use of monoclonal antibody therapy in the setting of a COVID-19 outbreak at a skilled nursing facility. Additionally, this paper reaffirms that use of monoclonal antibody therapies in persons with mild-to-moderate COVID-19 prevents severe outcomes such as death.
Author contributions: All authors contributed to conception of this manuscript, and from that start point, shared in writing and revision process.
Sponsors Role: No funding was received associated with this manuscript. There were no sponsors involved in the conceptualization or production of this manuscript.
Accepted Article This article is protected by copyright. All rights reserved. N/A cannot be calculated due too few of observations.
Accepted Article Accepted Article
References
Alam, Mahmud, Aggarwal, Clinical Impact of the Early Use of Monoclonal Antibody LY-CoV555 (Bamlanivimab) on Mortality and Hospitalization Among Elderly Nursing Home Patients: A Multicenter Retrospective Study, Cureus, doi:10.7759/cureus.14933
Bagchi, Mak, Li, Rates of COVID-19 among residents and staff members in nursing homes-United States, Morbidity and Mortality Weekly Report
Bariola, Mccreary, Wadas, Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection
Cdc, SARS-CoV-2 Variant Classifications and Definitions
Cohen, Nirula, Mulligan, Effect of Bamlanivimab vs Placebo on Incidence of COVID-19 Among Residents and Staff of Skilled Nursing and Assisted Living Facilities: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.8828
Dale, Hudson, Cullen, Administration of Bamlanivimab to Skilled Nursing Facility Residents during a COVID-19 Outbreak, Journal of the American Medical Directors Association
Garg, Kim, Whitaker, Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019-COVID-NET, 14 States
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, Jama
Harris, Taylor, Minor, The REDCap consortium: Building an international community of software platform partners, Journal of biomedical informatics
Harris, Taylor, Thielke, Payne, Gonzalez et al., A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hoffmann, Arora, Groß, SARS-CoV-2 variants B. 1.351 and P. 1 escape from neutralizing antibodies, Cell
Hosmer, Lemesbow, Goodness of fit tests for the multiple logistic regression model, Communications in statistics-Theory and Methods
Ko, Danielson, Town, Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system
Kumar, Wu, Stosor, Real-World Experience of Bamlanivimab for COVID-19: A Case-Control Study, Clinical Infectious Diseases
Mcmichael, Currie, Clark, Epidemiology of Covid-19 in a long-term care facility in King County, Washington, New England Journal of Medicine
Patel, Chaisson, Borgetti, Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility, Clinical Infectious Diseases
Williamson, Walker, Bhaskaran, Factors associated with COVID-19-related death using OpenSAFELY, Nature
DOI record:
{
"DOI": "10.1111/jgs.17705",
"ISSN": [
"0002-8614",
"1532-5415"
],
"URL": "http://dx.doi.org/10.1111/jgs.17705",
"alternative-id": [
"10.1111/jgs.17705"
],
"archive": [
"Portico"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-11-23"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-01-23"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-02-09"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1891-919X",
"affiliation": [
{
"name": "Epidemic Intelligence Service, Centers for Disease Control and Prevention"
},
{
"name": "Arizona Department of Health Services"
}
],
"authenticated-orcid": false,
"family": "Dale",
"given": "Ariella P.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Epidemic Intelligence Service, Centers for Disease Control and Prevention"
}
],
"family": "Hudson",
"given": "Matthew J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Devon Gables Rehabilitation Center"
}
],
"family": "Armenta",
"given": "Darunee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Devon Gables Rehabilitation Center"
}
],
"family": "Friebus",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Arizona"
}
],
"family": "Ellingson",
"given": "Katherine D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pima County Department of Public Health"
}
],
"family": "Davis",
"given": "Kat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pima County Department of Public Health"
}
],
"family": "Cullen",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Arizona Department of Health Services"
}
],
"family": "Brady",
"given": "Shane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Arizona Department of Health Services"
}
],
"family": "Komatsu",
"given": "Kenneth K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centers for Disease Control and Prevention COVID‐19 Response Team"
}
],
"family": "Stone",
"given": "Nimalie D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centers for Disease Control and Prevention COVID‐19 Response Team"
}
],
"family": "Uyeki",
"given": "Timothy M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centers for Disease Control and Prevention COVID‐19 Response Team"
}
],
"family": "Slifka",
"given": "Kara Jacobs",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Arizona"
},
{
"name": "Pima County Department of Public Health"
}
],
"family": "Perez‐Velez",
"given": "Carlos M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centers for Disease Control and Prevention COVID‐19 Response Team"
}
],
"family": "Keaton",
"given": "Amelia A.",
"sequence": "additional"
}
],
"container-title": [
"Journal of the American Geriatrics Society"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T07:18:37Z",
"timestamp": 1644477517000
},
"deposited": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T07:18:39Z",
"timestamp": 1644477519000
},
"indexed": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T07:42:03Z",
"timestamp": 1644478923419
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0002-8614"
},
{
"type": "electronic",
"value": "1532-5415"
}
],
"issued": {
"date-parts": [
[
2022,
2,
9
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T00:00:00Z",
"timestamp": 1644364800000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
9
]
],
"date-time": "2022-02-09T00:00:00Z",
"timestamp": 1644364800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jgs.17705",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jgs.17705",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2022,
2,
9
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
9
]
]
},
"publisher": "Wiley",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"J American Geriatrics Society"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Geriatrics and Gerontology"
],
"subtitle": [],
"title": [
"Clinical Outcomes of Monoclonal Antibody Therapy During a\n COVID\n ‐19 Outbreak in a Skilled Nursing Facility‐Arizona, 2021"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
