Inhaled Nitric Oxide via High-Flow Nasal Cannula in Patients with Acute Respiratory Failure Related to COVID-19
et al., Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine, doi:10.1177/11795484211047065, Jan 2021
43rd treatment shown to reduce risk in
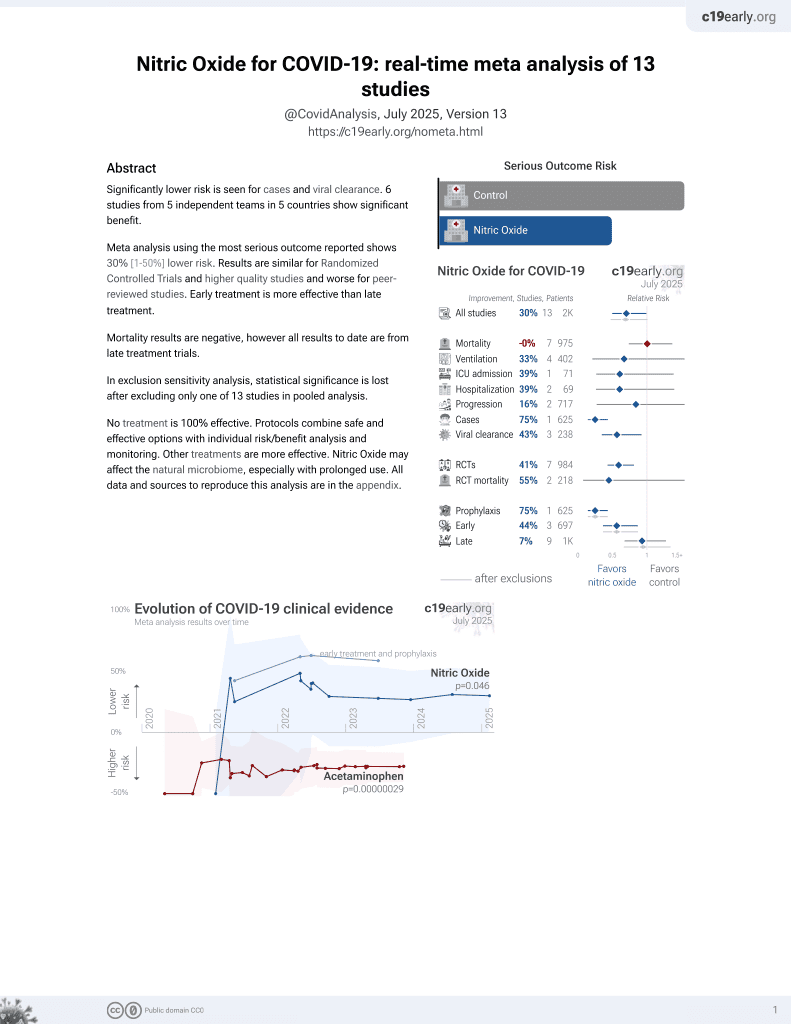
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 272 acute respiratory failure patients in the USA treated with high-flow nasal cannula, 66 treated with inhaled nitric oxide, showing increased mortality with inhaled nitric oxide. There were significant differences in the usage of several other treatments between the groups.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 54.1% higher, RR 1.54, p = 0.25, treatment 12 of 66 (18.2%), control 36 of 206 (17.5%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
risk of mechanical ventilation, 27.2% higher, RR 1.27, p = 0.26, treatment 29 of 66 (43.9%), control 79 of 206 (38.3%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chandel et al., 31 Jan 2021, retrospective, USA, peer-reviewed, 14 authors, study period 1 March, 2020 - 9 June, 2020.
Contact: abhimanyu.chandel.mil@mail.mil.
Inhaled Nitric Oxide via High-Flow Nasal Cannula in Patients with Acute Respiratory Failure Related to COVID-19
Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine, doi:10.1177/11795484211047065
INTRODUCTION: Limited evidence exists regarding use of inhaled nitric oxide (iNO) in spontaneously breathing patients. We evaluated the effectiveness of continuous iNO via high-flow nasal cannula (HFNC) in COVID-19 respiratory failure.
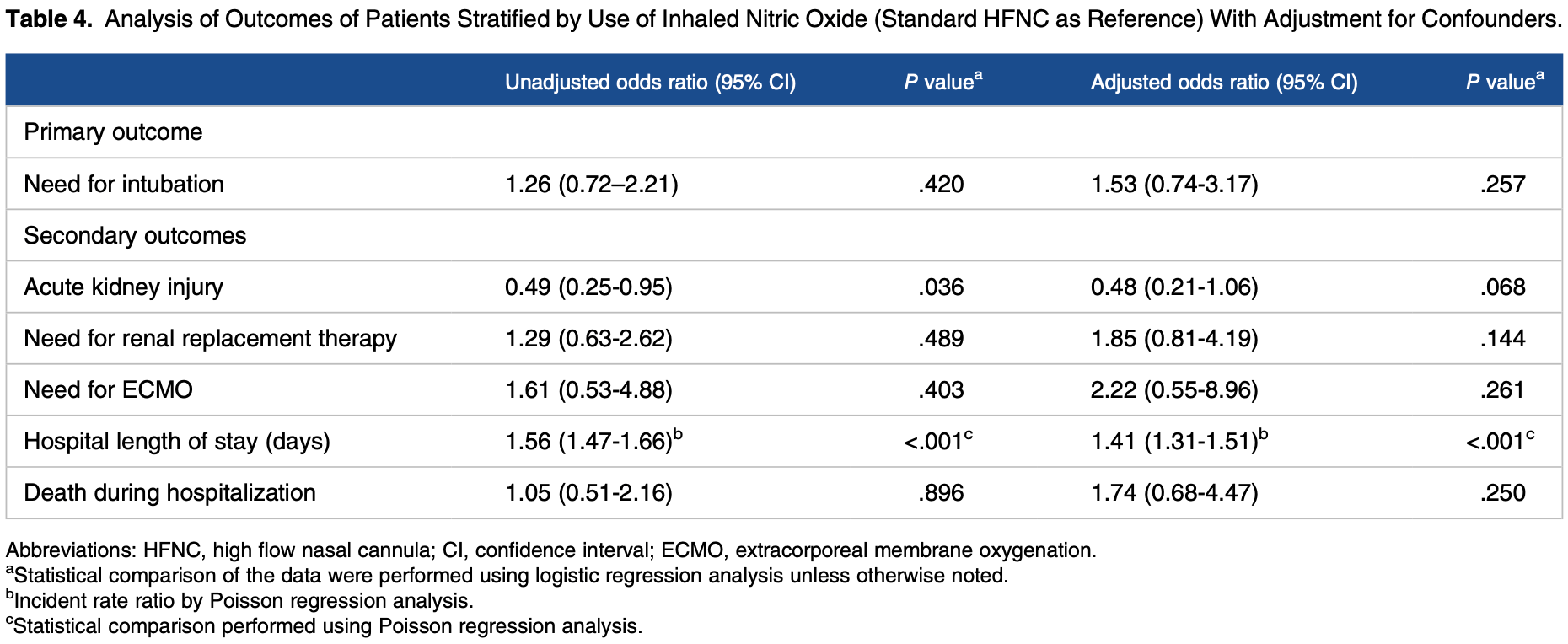
METHODS: We performed a multicenter cohort study of patients with respiratory failure from COVID-19 managed with HFNC. Patients were stratified by administration of iNO via HFNC. Regression analysis was used to compare the need for mechanical ventilation and secondary endpoints including hospital mortality, length of stay, acute kidney injury, need for renal replacement therapy, and need for extracorporeal life support. RESULTS: A total of 272 patients were identified and 66 (24.3%) of these patients received iNO via HFNC for a median of 88 h (interquartile range: 44, 135). After 12 h of iNO, supplemental oxygen requirement was unchanged or increased in 52.7% of patients. Twenty-nine (43.9%) patients treated with iNO compared to 79 (38.3%) patients without iNO therapy required endotracheal intubation (P = .47). After multivariable adjustment, there was no difference in need for mechanical ventilation between groups (odds ratio: 1.53; 95% confidence interval [CI]: 0.74-3.17), however, iNO administration was associated with longer hospital length of stay (incidence rate ratio: 1.41; 95% CI: 1.31-1.51). No difference was found for mortality, acute kidney injury, need for renal replacement therapy, or need for extracorporeal life support.
CONCLUSION: In patients with COVID-19 respiratory failure, iNO delivered via HFNC did not reduce oxygen requirements in the majority of patients or improve clinical outcomes. Given the observed association with increased length of stay, judicious selection of those likely to benefit from this therapy is warranted.
Author Contributions AC and CSK are the guarantors of the content of the manuscript and contributed to all aspects of the project. SP, KA, SA, AWB, DS, VK, OAS, AS, AWH, MD, and SDN contributed substantially to project design, data collection, and
ORCID iD Abhimanyu Chandel https://orcid.org/0000-0003-4879-1983
References
Abou-Arab, Huette, Debouvries, Inhaled nitric oxide for critically ill Covid-19 patients: a prospective study, Crit Care, doi:10.1186/s13054-020-03371-x
Adhikari, Dellinger, Lundin, Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis, Crit Care Med, doi:10.1097/CCM.0b013e3182a27909
Akerstrom, Mousavi-Jazi, Klingstrom, Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus, J Virol, doi:10.1128/JVI.79.3.1966-1969.2005
Bagate, Tuffet, Masi, Rescue therapy with inhaled nitric oxide and almitrine in COVID-19 patients with severe acute respiratory distress syndrome, Ann Intensive Care, doi:10.1186/s13613-020-00769-2
Berger, Kunichoff, Adhikari, Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.120.314872
Chandel, Patolia, Brown, High-flow nasal cannula in COVID-19: outcomes of application and examination of the ROX index to predict success, Respir Care, doi:10.4187/respcare.08631
Chang, Elhusseiny, Yeh, COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and meta-analysis, PLoS ONE, doi:10.1371/journal.pone.0246318
Davis, Crow, Fan, Use and costs of inhaled nitric oxide and inhaled epoprostenol in adult critically ill patients: a quality improvement project, Am J Health Syst Pharm, doi:10.1093/ajhp/zxz151
Dellinger, Zimmerman, Taylor, Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled nitric oxide in ARDS study group, Crit Care Med, doi:10.1097/00003246-199801000-00011
Demoule, Baron, Darmon, High-flow nasal cannula in critically III patients with severe COVID-19, Am J Respir Crit Care Med, doi:10.1164/rccm.202005-2007LE
Ely, Shintani, Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS), JAMA, doi:10.1001/jama.289.22.2983
Fang, Jiang, Su, The role of NO in COVID-19 and potential therapeutic strategies, Free Radic Biol Med, doi:10.1016/j.freeradbiomed.2020.12.008
Ferrari, Santini, Protti, Inhaled nitric oxide in mechanically ventilated patients with COVID-19, J Crit Care, doi:10.1016/j.jcrc.2020.08.007
Gattinoni, Coppola, Cressoni, COVID-19 does not lead to a "typical" acute respiratory distress syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.202003-0817LE
Gebistorf, Karam, Wetterslev, Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults, Cochrane Database Syst Rev, doi:10.1002/14651858.CD002787.pub3
Griffiths, Evans, Inhaled nitric oxide therapy in adults, N Engl J Med, doi:10.1056/NEJMra051884
Kline, Puskarich, Jones, Inhaled nitric oxide to treat intermediate risk pulmonary embolism: a multicenter randomized controlled trial, Nitric Oxide, doi:10.1016/j.niox.2019.01.006
Lang, Som, Mendoza, Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT, Lancet Infect Dis, doi:10.1016/s1473-3099(20)30367-4
Longobardo, Montanari, Shulman, Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome, Br J Anaesth, doi:10.1016/j.bja.2020.10.011
Nishimura, High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects, Respir Care, doi:10.4187/respcare.04577
Parikh, Wilson, Weinberg, Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients, Ther Adv Respir Dis, doi:10.1177/1753466620933510
Reynolds, Lee, Renz, Pulmonary vascular dilatation detected by automated transcranial Doppler in COVID-19 pneumonia, Am J Respir Crit Care Med, doi:10.1164/rccm.202006-2219LE
Saunders, Davis, Year in review: pharmacologic treatments for COVID-19, Respir Care, doi:10.4187/respcare.09153
Tavazzi, Pozzi, Mongodi, Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia, Crit Care, doi:10.1186/s13054-020-03222-9
Therapeutics, Bellerophon Therapeutics Announces Results of Interim Analysis of Phase 3 COViNOX Study of INOpulse® for the Treatment of COVID-19
Tzanetos, Housley, Barr, Implementation of an inhaled nitric oxide protocol decreases direct cost associated with its use, Respir Care, doi:10.4187/respcare.03308
Wiegand, Fakhr, Carroll, Rescue treatment with high-dose gaseous nitric oxide in spontaneously breathing patients with severe coronavirus disease 2019, Crit Care Explor, doi:10.1097/cce.0000000000000277
DOI record:
{
"DOI": "10.1177/11795484211047065",
"ISSN": [
"1179-5484",
"1179-5484"
],
"URL": "http://dx.doi.org/10.1177/11795484211047065",
"abstract": "<jats:sec><jats:title>INTRODUCTION</jats:title><jats:p> Limited evidence exists regarding use of inhaled nitric oxide (iNO) in spontaneously breathing patients. We evaluated the effectiveness of continuous iNO via high-flow nasal cannula (HFNC) in COVID-19 respiratory failure. </jats:p></jats:sec><jats:sec><jats:title>METHODS</jats:title><jats:p> We performed a multicenter cohort study of patients with respiratory failure from COVID-19 managed with HFNC. Patients were stratified by administration of iNO via HFNC. Regression analysis was used to compare the need for mechanical ventilation and secondary endpoints including hospital mortality, length of stay, acute kidney injury, need for renal replacement therapy, and need for extracorporeal life support. </jats:p></jats:sec><jats:sec><jats:title>RESULTS</jats:title><jats:p> A total of 272 patients were identified and 66 (24.3%) of these patients received iNO via HFNC for a median of 88 h (interquartile range: 44, 135). After 12 h of iNO, supplemental oxygen requirement was unchanged or increased in 52.7% of patients. Twenty-nine (43.9%) patients treated with iNO compared to 79 (38.3%) patients without iNO therapy required endotracheal intubation ( P = .47). After multivariable adjustment, there was no difference in need for mechanical ventilation between groups (odds ratio: 1.53; 95% confidence interval [CI]: 0.74-3.17), however, iNO administration was associated with longer hospital length of stay (incidence rate ratio: 1.41; 95% CI: 1.31-1.51). No difference was found for mortality, acute kidney injury, need for renal replacement therapy, or need for extracorporeal life support. </jats:p></jats:sec><jats:sec><jats:title>CONCLUSION</jats:title><jats:p> In patients with COVID-19 respiratory failure, iNO delivered via HFNC did not reduce oxygen requirements in the majority of patients or improve clinical outcomes. Given the observed association with increased length of stay, judicious selection of those likely to benefit from this therapy is warranted. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/11795484211047065"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-4879-1983",
"affiliation": [
{
"name": "Walter Reed National Military Medical Center, Bethesda, MD, USA"
}
],
"authenticated-orcid": false,
"family": "Chandel",
"given": "Abhimanyu",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Virginia Commonwealth University School of Medicine, Richmond, VA, USA"
}
],
"family": "Patolia",
"given": "Saloni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Ahmad",
"given": "Kareem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Aryal",
"given": "Shambhu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Brown",
"given": "A Whitney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Sahjwani",
"given": "Dhwani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Khangoora",
"given": "Vikramjit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Shlobin",
"given": "Oksana A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Cameron",
"given": "Paula C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Singhal",
"given": "Anju",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Walter Reed National Military Medical Center, Bethesda, MD, USA"
}
],
"family": "Holtzclaw",
"given": "Arthur W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Desai",
"given": "Mehul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "Nathan",
"given": "Steven D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Inova Fairfax Hospital, Falls Church, VA, USA"
}
],
"family": "King",
"given": "Christopher S",
"sequence": "additional"
}
],
"container-title": "Clinical Medicine Insights: Circulatory, Respiratory and Pulmonary Medicine",
"container-title-short": "Clin Med Insights Circ Respir Pulm Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
29
]
],
"date-time": "2021-09-29T14:20:54Z",
"timestamp": 1632925254000
},
"deposited": {
"date-parts": [
[
2021,
9,
29
]
],
"date-time": "2021-09-29T14:20:58Z",
"timestamp": 1632925258000
},
"indexed": {
"date-parts": [
[
2022,
6,
5
]
],
"date-time": "2022-06-05T00:51:42Z",
"timestamp": 1654390302248
},
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2021,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/11795484211047065",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/11795484211047065",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/11795484211047065",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "117954842110470",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
29
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1164/rccm.202003-0817LE",
"doi-asserted-by": "publisher",
"key": "bibr1-11795484211047065"
},
{
"DOI": "10.1371/journal.pone.0246318",
"doi-asserted-by": "publisher",
"key": "bibr2-11795484211047065"
},
{
"DOI": "10.1164/rccm.202005-2007LE",
"doi-asserted-by": "publisher",
"key": "bibr3-11795484211047065"
},
{
"DOI": "10.4187/respcare.04577",
"doi-asserted-by": "publisher",
"key": "bibr4-11795484211047065"
},
{
"author": "Chandel A",
"journal-title": "Respir Care",
"key": "bibr5-11795484211047065",
"year": "2020"
},
{
"DOI": "10.4187/respcare.09153",
"doi-asserted-by": "publisher",
"key": "bibr6-11795484211047065"
},
{
"DOI": "10.1097/00003246-199801000-00011",
"doi-asserted-by": "publisher",
"key": "bibr7-11795484211047065"
},
{
"DOI": "10.1056/NEJMra051884",
"doi-asserted-by": "publisher",
"key": "bibr8-11795484211047065"
},
{
"DOI": "10.1097/CCM.0b013e3182a27909",
"doi-asserted-by": "publisher",
"key": "bibr9-11795484211047065"
},
{
"author": "Gebistorf F",
"first-page": "CD002787",
"issue": "6",
"journal-title": "Cochrane Database Syst Rev",
"key": "bibr10-11795484211047065",
"year": "2016"
},
{
"DOI": "10.1093/ajhp/zxz151",
"doi-asserted-by": "publisher",
"key": "bibr11-11795484211047065"
},
{
"DOI": "10.4187/respcare.03308",
"doi-asserted-by": "publisher",
"key": "bibr12-11795484211047065"
},
{
"DOI": "10.1128/JVI.79.3.1966-1969.2005",
"doi-asserted-by": "publisher",
"key": "bibr13-11795484211047065"
},
{
"DOI": "10.1016/j.freeradbiomed.2020.12.008",
"doi-asserted-by": "publisher",
"key": "bibr14-11795484211047065"
},
{
"DOI": "10.1186/s13613-020-00769-2",
"doi-asserted-by": "publisher",
"key": "bibr15-11795484211047065"
},
{
"DOI": "10.1016/j.bja.2020.10.011",
"doi-asserted-by": "publisher",
"key": "bibr16-11795484211047065"
},
{
"DOI": "10.1016/j.jcrc.2020.08.007",
"doi-asserted-by": "publisher",
"key": "bibr17-11795484211047065"
},
{
"DOI": "10.1177/1753466620933510",
"doi-asserted-by": "publisher",
"key": "bibr18-11795484211047065"
},
{
"DOI": "10.1186/s13054-020-03222-9",
"doi-asserted-by": "publisher",
"key": "bibr19-11795484211047065"
},
{
"DOI": "10.1186/s13054-020-03371-x",
"doi-asserted-by": "publisher",
"key": "bibr20-11795484211047065"
},
{
"DOI": "10.1097/CCE.0000000000000277",
"doi-asserted-by": "publisher",
"key": "bibr21-11795484211047065"
},
{
"DOI": "10.1001/jama.289.22.2983",
"doi-asserted-by": "publisher",
"key": "bibr23-11795484211047065"
},
{
"DOI": "10.1161/ATVBAHA.120.314872",
"doi-asserted-by": "publisher",
"key": "bibr24-11795484211047065"
},
{
"DOI": "10.1016/j.niox.2019.01.006",
"doi-asserted-by": "publisher",
"key": "bibr25-11795484211047065"
},
{
"DOI": "10.1016/S1473-3099(20)30367-4",
"doi-asserted-by": "publisher",
"key": "bibr26-11795484211047065"
},
{
"DOI": "10.1164/rccm.202006-2219LE",
"doi-asserted-by": "publisher",
"key": "bibr27-11795484211047065"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/11795484211047065"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cardiology and Cardiovascular Medicine",
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Inhaled Nitric Oxide via High-Flow Nasal Cannula in Patients with Acute Respiratory Failure Related to COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "15"
}