Metabolic Reprogramming in Respiratory Viral Infections: A Focus on SARS-CoV-2, Influenza, and Respiratory Syncytial Virus
et al., Biomolecules, doi:10.3390/biom15071027, Jul 2025
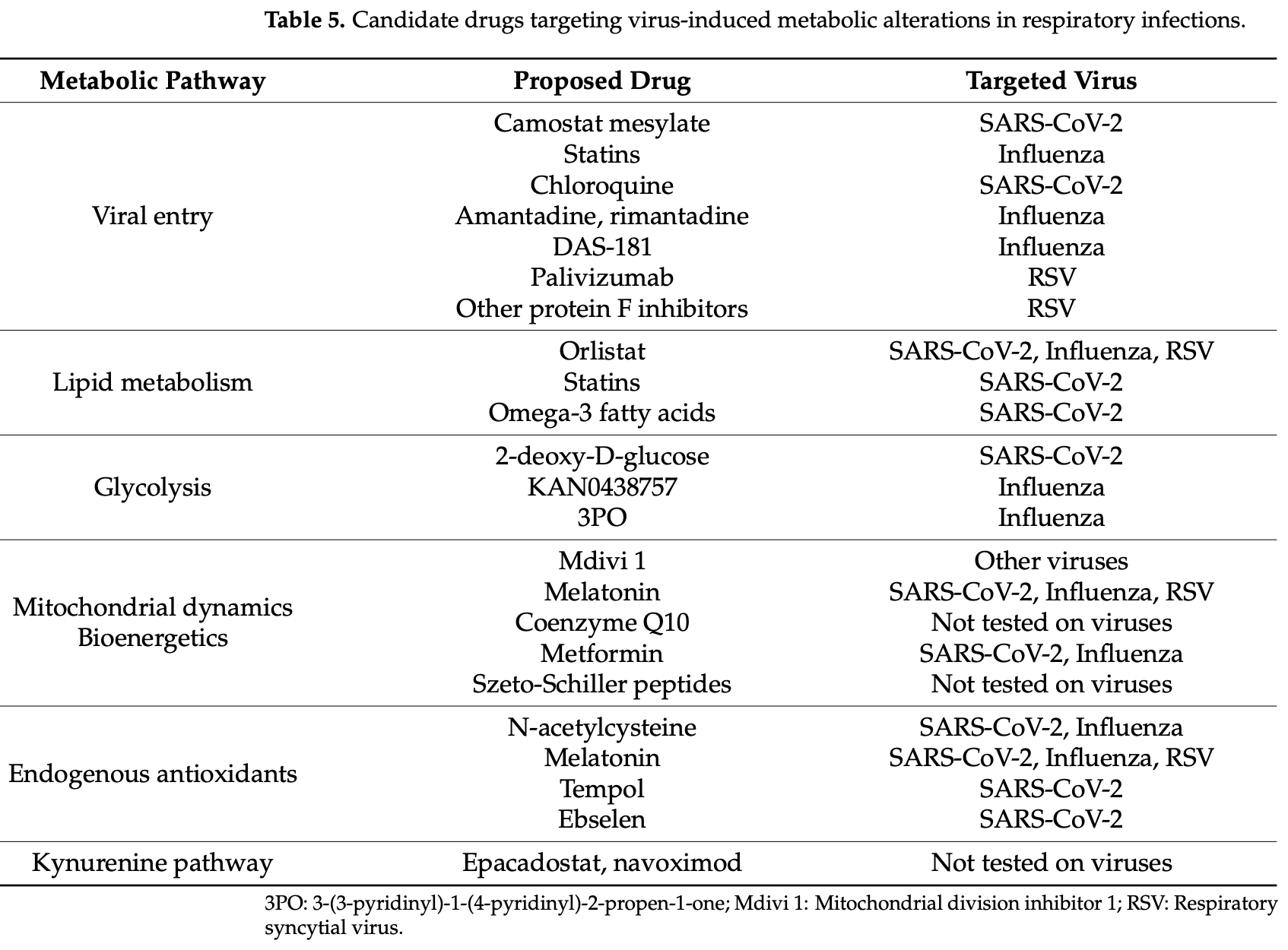
Review of metabolic reprogramming in respiratory viral infections, focusing on SARS-CoV-2, influenza, and respiratory syncytial virus. Authors describe how these viruses exploit host cellular metabolism to support their replication, modulate immune responses, and promote disease progression. SARS-CoV-2 induces a shift toward glycolysis (Warburg-like effect) while suppressing oxidative phosphorylation, providing the energy and biosynthetic precursors needed for viral replication. The virus also disrupts lipid metabolism, enhancing fatty acid synthesis and altering cholesterol homeostasis to facilitate viral entry, replication, and immune evasion. Lipid rafts serve as platforms for SARS-CoV-2 attachment and entry through ACE2 receptor binding. Authors detail how the virus induces lipid droplet accumulation, impairs fatty acid oxidation, and causes significant alterations in circulating lipoproteins. SARS-CoV-2 infection also causes mitochondrial dysfunction, promoting mitochondrial fragmentation through Drp1 activation and impairing mitochondrial antiviral signaling. The virus manipulates key metabolic regulators like mTOR and AMPK to create a pro-viral state and alters amino acid metabolism, particularly glutamine, arginine, tryptophan, and cysteine pathways. Authors highlight how these metabolic disruptions contribute to oxidative stress and inflammation, with depletion of glutathione and reduced paraoxonase-1 activity correlating with disease severity. The review identifies promising therapeutic targets based on these metabolic alterations, including glycolysis inhibitors, lipid metabolism modulators, antioxidants, and mitochondrial protectants.
Camps et al., 16 Jul 2025, multiple countries, peer-reviewed, 5 authors.
Contact: jorge.camps@salutsantjoan.cat (corresponding author), andrea.jimenez@urv.cat, jorge.joven@salutsantjoan.cat, simona.mihaela@salutsantjoan.cat, antoni.castro@urv.cat.
Metabolic Reprogramming in Respiratory Viral Infections: A Focus on SARS-CoV-2, Influenza, and Respiratory Syncytial Virus
Biomolecules, doi:10.3390/biom15071027
Respiratory infections caused by severe acute respiratory syndrome coronavirus 2, influenza virus, and respiratory syncytial virus pose significant global health challenges, leading to high morbidity and mortality, particularly in vulnerable populations. Despite their distinct virological characteristics, these viruses exploit host cellular metabolism to support replication, modulate immune responses, and promote disease progression. Emerging evidence shows that they induce metabolic reprogramming, shifting cellular energy production toward glycolysis to meet the bioenergetic demands of viral replication. Additionally, alterations in lipid metabolism, including enhanced fatty acid synthesis and disrupted cholesterol homeostasis, facilitate viral entry, replication, and immune evasion. The dysregulation of mitochondrial function and oxidative stress pathways also contributes to disease severity and long-term complications, such as persistent inflammation and immune exhaustion. Understanding these metabolic shifts is crucial for identifying new therapeutic targets and novel biomarkers for early disease detection, prognosis, and patient stratification. This review provides an overview of the metabolic alterations induced by severe acute respiratory syndrome coronavirus 2, influenza virus, and respiratory syncytial virus, highlighting shared and virus-specific mechanisms and potential therapeutic interventions.
Author Contributions: Conceptualization, J.C., S.I., A.C. and J.J.; investigation, J.C., S.I. and A.J.-F.; validation, J.C.; visualization, J.C. and S.I.; writing-original draft preparation, J.C. and S.I.; writingreview and editing, J.C., S.I., A.C. and J.J. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest: The authors declare no conflicts of interest.
Abbreviations
ACE2 Angiotensin-converting enzyme
References
Afrose, Junaid, Akter, Tania, Zheng et al., Targeting kinases with thymoquinone: A molecular approach to cancer therapeutics, Drug Discov. Today, doi:10.1016/j.drudis.2020.07.019
Agus, Planchais, Sokol, Gut microbiota regulation of tryptophan metabolism in health and disease, Cell Host Microbe, doi:10.1016/j.chom.2018.05.003
Alberti, Gladfelter, Mittag, Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates, Cell, doi:10.1016/j.cell.2018.12.035
Alenquer, Vale-Costa, Etibor, Ferreira, Sousa et al., Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites, Nat. Commun, doi:10.1038/s41467-019-09549-4
Ali, Ben-Sahra, Regulation of nucleotide metabolism in cancers and immune disorders, Trends Cell Biol, doi:10.1016/j.tcb.2023.03.003
Aliyari, Ghaffari, Pernet, Parvatiyar, Wang et al., Suppressing fatty acid synthase by type I interferon and chemical inhibitors as a broad spectrum anti-viral strategy against SARS-CoV-2, Acta Pharm. Sin. B, doi:10.1016/j.apsb.2022.02.019
Allen, Arjona, Santerre, Sawaya, Hallmarks of metabolic reprogramming and their role in viral pathogenesis, Viruses, doi:10.3390/v14030602
Amatore, Sgarbanti, Aquilano, Baldelli, Limongi et al., Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS, Cell. Microbiol, doi:10.1111/cmi.12343
Amigó, Martínez-Micaelo, Velasco, Casas, Correig et al., Identification of a molecular signature associated with covid-19 severity using a comprehensive 1H-NMR serum metabolomics profiling strategy, Atherosclerosis, doi:10.1016/j.atherosclerosis.2024.117832
Ammer, Nietzsche, Rien, Kühnl, Mader et al., The anti-obesity drug orlistat reveals anti-viral activity, Med. Microbiol. Immunol, doi:10.1007/s00430-015-0391-4
Anand, Mande, Diet, microbiota and gut-lung connection, Front. Microbiol, doi:10.3389/fmicb.2018.02147
Andersson, Ottestad, Tracey, Extracellular HMGB1: A therapeutic target in severe pulmonary inflammation including COVID-19?, Mol. Med, doi:10.1186/s10020-020-00172-4
Archer, Dasgupta, Chen, Wu, Baid et al., SARS-CoV-2 mitochondriopathy in COVID-19 pneumonia exacerbates hypoxemia, Redox Biol, doi:10.1016/j.redox.2022.102508
Arnardottir, Pawelzik, Öhlund Wistbacka, Artiach, Hofmann et al., Stimulating the resolution of inflammation through omega-3 polyunsaturated fatty acids in COVID-19: Rationale for the COVID-Omega-F trial, Front. Physiol, doi:10.3389/fphys.2020.624657
Assimakopoulos, Aretha, Komninos, Dimitropoulou, Lagadinou et al., N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: A two-center retrospective cohort study, Infect. Dis, doi:10.1080/23744235.2021.1945675
Ayres, A metabolic handbook for the COVID-19 pandemic, Nat. Metab, doi:10.1038/s42255-020-0237-2
Bahmyari, Zare, Sharma, Agarwal, Halvaei, The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis, Andrologia, doi:10.1111/and.13514
Bahrampour Juybari, Pourhanifeh, Hosseinzadeh, Hemati, Mehrzadi, Melatonin potentials against viral infections including COVID-19: Current evidence and new findings, Virus Res, doi:10.1016/j.virusres.2020.198108
Baiges-Gaya, Iftimie, Castañé, Rodríguez-Tomàs, Jiménez-Franco et al., Combining semi-targeted metabolomics and machine learning to identify metabolic alterations in the serum and urine of hospitalized patients with COVID-19, Biomolecules, doi:10.3390/biom13010163
Ballout, Kong, Sampson, Otvos, Cox et al., The NIH lipo-COVID study: A pilot NMR investigation of lipoprotein subfractions and other metabolites in patients with severe COVID-19, Biomedicines, doi:10.3390/biomedicines9091090
Barberis, Timo, Amede, Vanella, Puricelli et al., Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2, Int. J. Mol. Sci, doi:10.3390/ijms21228623
Barbier, Lang, Valois, Rothman, Medin, Dengue virus induces mitochondrial elongation through impairment of Drp1-triggered mitochondrial fission, Virology, doi:10.1016/j.virol.2016.10.022
Barrett, Dutch, Viral membrane fusion and the transmembrane domain, Viruses, doi:10.3390/v12070693
Basturea, Endocytosis, None, Mater. Methods, doi:10.13070/mm.en.9.2752
Berta, Zsíros, Bodor, Balogh, Lőrincz et al., Clinical aspects of genetic and non-genetic cardiovascular risk factors in familial hypercholesterolemia, Genes, doi:10.3390/genes13071158
Bhatt, Shenoy, Munjal, Chinnadurai, Agarwal et al., 2-deoxy-D-glucose as an adjunct to standard of care in the medical management of COVID-19: A proof-of-concept and dose-ranging randomised phase II clinical trial, BMC Infect. Dis, doi:10.1186/s12879-022-07642-6
Bhol, Bhanjadeo, Singh, Dash, Ojha et al., The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anticytokine compounds, Biomed. Pharmacother, doi:10.1016/j.biopha.2024.117177
Bhutta, Gallo, Borenstein, Multifaceted role of AMPK in viral infections, Cells, doi:10.3390/cells10051118
Bizkarguenaga, Bruzzone, Gil-Redondo, Sanjuan, Martin-Ruiz et al., Uneven metabolic and lipidomic profiles in recovered COVID-19 patients as investigated by plasma NMR metabolomics, NMR Biomed, doi:10.1002/nbm.4637
Blevins, Xu, Biby, Zhang, The NLRP3 inflammasome pathway: A review of mechanisms and inhibitors for the treatment of inflammatory diseases, Front. Aging Neurosci, doi:10.3389/fnagi.2022.879021
Bogan, Brenner, Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition, Annu. Rev. Nutr, doi:10.1146/annurev.nutr.28.061807.155443
Bohdanowicz, Grinstein, Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis, Physiol. Rev, doi:10.1152/physrev.00002.2012
Bojkova, Costa, Reus, Bechtel, Jaboreck et al., Targeting the pentose phosphate pathway for SARS-CoV-2 therapy, Metabolites, doi:10.3390/metabo11100699
Bolland, Marechal, Petiot, Porrot, Guivel-Benhassine et al., SARS-CoV-2 entry and fusion are independent of ACE2 localization to lipid rafts, J. Virol, doi:10.1128/jvi.01823-24
Boyd, Brookfield, Critchlow, Cumming, Curtis et al., Structure-based design of potent and selective inhibitors of the metabolic kinase PFKFB3, J. Med. Chem, doi:10.1021/acs.jmedchem.5b00352
Bruzzone, Bizkarguenaga, Gil-Redondo, Diercks, Arana et al., SARS-CoV-2 infection dysregulates the metabolomic and lipidomic profiles of serum, iScience, doi:10.1016/j.isci.2020.101645
Bukrinsky, Mukhamedova, Sviridov, Lipid rafts and pathogens: The art of deception and exploitation, J. Lipid Res, doi:10.1194/jlr.TR119000391
Camps, Castañé, Rodríguez-Tomàs, Baiges-Gaya, Hernández-Aguilera et al., On the role of paraoxonase-1 and chemokine ligand 2 (C-C motif) in metabolic alterations linked to inflammation and disease. A 2021 update, Biomolecules, doi:10.3390/biom11070971
Camps, Iftimie, Arenas, Castañé, Jiménez-Franco et al., Paraoxonase-1: How a xenobiotic detoxifying enzyme has become an actor in the pathophysiology of infectious diseases and cancer, Chem. Biol. Interact, doi:10.1016/j.cbi.2023.110553
Camps, Iftimie, García-Heredia, Castro, Joven, Paraoxonases and infectious diseases, Clin. Biochem, doi:10.1016/j.clinbiochem.2017.04.016
Camps, Jiménez-Franco, García-Pablo, Joven, Arenas, Artificial intelligence-driven integration of multi-omics and radiomics: A new hope for precision cancer diagnosis and prognosis, Biochim. Biophys. Acta Mol. Basis Dis, doi:10.1016/j.bbadis.2025.167841
Camps, Marsillach, Joven, Pharmacological and lifestyle factors modulating serum paraoxonase-1 activity, Mini Rev. Med. Chem, doi:10.2174/138955709788681591
Camps, Rodríguez-Gallego, García-Heredia, Triguero, Riera-Borrull et al., Paraoxonases and chemokine (C-C motif) ligand-2 in noncommunicable diseases, Adv. Clin. Chem, doi:10.1016/b978-0-12-800094-6.00007-8
Carter, Iqbal, The influenza A virus replication cycle: A comprehensive review, Viruses, doi:10.3390/v16020316
Castañé, Iftimie, Baiges-Gaya, Rodríguez-Tomàs, Jiménez-Franco et al., Machine learning and semi-targeted lipidomics identify distinct serum lipid signatures in hospitalized COVID-19-positive and COVID-19-negative patients, Metabolism, doi:10.1016/j.metabol.2022.155197
Catapano, Pirillo, Bonacina, Norata, HDL in innate and adaptive immunity, Cardiovasc. Res, doi:10.1093/cvr/cvu150
Cecchini, Cecchini, SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression, Med. Hypotheses, doi:10.1016/j.mehy.2020.110102
Chakraborty, Veettil, Bottero, Chandran, Kaposi's sarcoma-associated herpesvirus interacts with EphrinA2 receptor to amplify signaling essential for productive infection, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1119592109
Chandel, Lipid metabolism, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a040576
Chau, Chen, Cochrane, Parent, Mouland, Liquid-liquid phase separation of nucleocapsid proteins during SARS-CoV-2 and HIV-1 replication, Cell Rep, doi:10.1016/j.celrep.2022.111968
Chawla, Subramanian, Rahman, Fan, Chakravarty et al., Autophagy in virus infection: A race between host immune response and viral antagonism, Immuno, doi:10.3390/immuno2010012
Chen, Aoki, Huang, Hirono, Chen et al., White spot Syndrome virus induces metabolic changes resembling the Warburg effect in shrimp hemocytes in the early stage of infection, J. Virol, doi:10.3390/cells11131982
Chen, Cai, Zhang, Tang, Chen et al., Respiratory syncytial virus co-opts hypoxia-inducible factor-1α-mediated glycolysis to favor the production of infectious virus, mBio, doi:10.1128/mbio.02110-23
Chen, Chen, Liang, Chen, Zhang et al., Potential role of superoxide dismutase 3 (SOD3) in resistance to Influenza A virus infection, Antioxidants, doi:10.3390/antiox12020354
Chen, Jiang, Gu, Yue, Liu et al., SARS-CoV-2 nucleocapsid protein interaction with YBX1 displays oncolytic properties through PKM mRNA destabilization, Mol. Cancer, doi:10.1186/s12943-024-02153-1
Chen, Niu, Lv, Multi-omics insights reveal the remodeling of gut mycobiome with P. gingivalis, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.937725
Chen, Shi, Sun, Zhou, Wang et al., Metformin alleviates inflammatory response and severity rate of COVID-19 infection in elderly individuals, Sci. Rep, doi:10.1038/s41598-025-96294-y
Chen, Zhang, Xu, Chen, Tang et al., Cholesterol-rich lysosomes induced by respiratory syncytial virus promote viral replication by blocking autophagy flux, Nat. Commun, doi:10.1038/s41467-024-50711-4
Chen, Zhao, Huang, Wen, Feng, Synergetic impact of combined navoximod with cisplatin mitigates chemo-immune resistance via blockading IDO1+ CAFs-secreted Kyn/AhR/IL-6 and pol ζ-prevented CIN in human oral squamous cell carcinoma, Life Sci, doi:10.1016/j.lfs.2023.122239
Cheng, Wang, Yin, Liang, Zhang et al., The nonstructural protein 1 of respiratory syncytial virus hijacks host mitophagy as a novel mitophagy receptor to evade the type I IFN response in HEp-2 cells, mBio, doi:10.1128/mbio.01480-23
Cheudjeu, Correlation of D-xylose with severity and morbidity-related factors of COVID-19 and possible therapeutic use of D-xylose and antibiotics for COVID-19, Life Sci, doi:10.1016/j.lfs.2020.118335
Cheung, Gill, Esposito, Kaminski, Courousse et al., Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication, J. Virol, doi:10.1128/JVI.01757-09
Cho, Kim, Lee, Kwon, Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity, Antioxidants, doi:10.3390/antiox10020209
Choi, Knobil, Otterbein, Eastman, Jacoby, Oxidant stress responses in influenza virus pneumonia: Gene expression and transcription factor activation, Am. J. Physiol, doi:10.1152/ajplung.1996.271.3.L383
Chu, Hua, Liu, Xiong, Jiang et al., Superoxide dismutase alterations in COVID-19: Implications for disease severity and mortality prediction in the context of omicron variant infection, Front. Immunol, doi:10.3389/fimmu.2024.1362102
Chu, Xing, Du, Duan, Liu et al., Pharmacological inhibition of fatty acid synthesis blocks SARS-CoV-2 replication, Nat. Metab, doi:10.1038/s42255-021-00479-4
Churiso, Husen, Bulbula, Abebe, Immunity cell responses to RSV and the role of antiviral inhibitors: A systematic review, Infect. Drug Resist, doi:10.2147/IDR.S387479
Cicin, Plimack, Gurney, Leibowitz, Alekseev et al., Epacadostat plus pembrolizumab versus placebo plus pembrolizumab for advanced urothelial carcinoma: Results from the randomized phase III ECHO-303/KEYNOTE-698 study, BMC Cancer, doi:10.1186/s12885-023-11213-6
Clem, O'neal, Tapolsky, Clem, Imbert-Fernandez et al., Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer, Mol. Cancer Ther, doi:10.1158/1535-7163.MCT-13-0097
Codo, Davanzo, Monteiro, De Souza, Muraro et al., Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent Axis, Cell Metab, doi:10.1016/j.cmet.2020.07.007
Colonetti, De Carvalho, Rangel, Pinto, Roesch et al., Are the bacteria and their metabolites contributing for gut inflammation on GSD-Ia patients?, Metabolites, doi:10.3390/metabo12090873
Corn, Lund, Andersson, Dohlmann, Hlatky et al., Low-density lipoprotein cholesterol response to statins according to comorbidities and co-medications: A population-based study, Am. Heart J, doi:10.1016/j.ahj.2024.04.018
Cruz-Pulido, Mounce, Good cop, bad cop: Polyamines play both sides in host immunity and viral replication, Semin. Cell Dev. Biol, doi:10.1016/j.semcdb.2022.12.004
Cruzat, Macedo Rogero, Noel Keane, Curi, Newsholme, Glutamine: Metabolism and immune function, supplementation and clinical translation, Nutrients, doi:10.3390/nu10111564
Cuyàs, Fernández-Arroyo, Verdura, García, Stursa et al., Metformin regulates global DNA methylation via mitochondrial one-carbon metabolism, Oncogene, doi:10.1038/onc.2017.367
Dai, Tang, Qi, Liu, Bajinka et al., Dispersion and utilization of lipid droplets mediates respiratory syncytial virus-induced airway hyperresponsiveness, Pediatr. Allergy Immunol, doi:10.1111/pai.13651
Dantonio, Klein, Freire, Araujo, Chiacetti et al., Exploring major signaling cascades in melanomagenesis: A rationale route for targetted skin cancer therapy, Biosci. Rep, doi:10.1042/BSR20180511
Darweesh, Mohammadi, Rahmati, Al-Hamadani, Al-Harrasi, Metabolic reprogramming in viral infections: The interplay of glucose metabolism and immune responses, Front. Immunol, doi:10.3389/fimmu.2025.1578202
Das, Chakraborty, Basu, Critical role of lipid rafts in virus entry and activation of phosphoinositide 3 ′ kinase/Akt signaling during early stages of Japanese encephalitis virus infection in neural stem/progenitor cells, J. Neurochem, doi:10.1111/j.1471-4159.2010.06951.x
De Angelis, Amatore, Checconi, Zevini, Fraternale et al., Influenza virus down-modulates G6PD expression and activity to induce oxidative stress and promote its replication, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.804976
De Clercq, Chemotherapy of respiratory syncytial virus infections: The final breakthrough, Int. J. Antimicrob Agents, doi:10.1016/j.ijantimicag.2014.12.025
De Flora, Grassi, Carati, Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment, Eur. Respir. J, doi:10.1183/09031936.97.10071535
De Vries, Tscherne, Wienholts, Cobos-Jiménez, Scholte et al., Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway, PLoS Pathog, doi:10.1371/journal.ppat.1001329
Dean, Ochoa, Sanchez-Pino, Zabaleta, Garai et al., Severe COVID-19 is characterized by an impaired type I interferon response and elevated levels of arginase producing granulocytic myeloid derived suppressor cells, Front. Immunol, doi:10.3389/fimmu.2021.695972
Dehhaghi, Heydari, Panahi, Lewin, Heng et al., The roles of the kynurenine pathway in COVID-19 neuropathogenesis, Infection, doi:10.1007/s15010-024-02293-y
Delafiori, Navarro, Siciliano, De Melo, Busanello et al., Covid-19 automated diagnosis and risk assessment through metabolomics and machine learning, Anal. Chem, doi:10.1021/acs.analchem.0c04497
Deretic, Autophagy in inflammation, infection, and immunometabolism, Immunity, doi:10.1016/j.immuni.2021.01.018
Dias, Soares, Ferreira, Sacramento, Fintelman-Rodrigues et al., Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators, PLoS Pathog, doi:10.1371/journal.ppat.1009127
Diaz-Arocutipa, Melgar-Talavera, Alvarado-Yarasca, Saravia-Bartra, Cazorla et al., Statins reduce mortality in patients with COVID-19: An updated meta-analysis of 147 824 patients, Int. J. Infect. Dis, doi:10.1016/j.ijid.2021.08.004
Diehl, Miettinen, Elbashir, Nabel, Darnell et al., Nucleotide imbalance decouples cell growth from cell proliferation, Nat. Cell Biol, doi:10.1038/s41556-022-00965-1
Ding, Robinson, Li, Aldhowayan, Geetha et al., Mitochondrial dysfunction and beneficial effects of mitochondria-targeted small peptide SS-31 in Diabetes Mellitus and Alzheimer's disease, Pharmacol. Res, doi:10.1016/j.phrs.2021.105783
Djuricic, Calder, Omega-3 (n-3) fatty acid-statin interaction: Evidence for a novel therapeutic strategy for atherosclerotic cardiovascular disease, Nutrients, doi:10.3390/nu16070962
Doyle, Goodson, Kolaczkowski, Liu, Jia et al., Manipulation of host cholesterol by SARS-CoV-2, bioRxiv, doi:10.1101/2024.11.13.623299
Duan, Liu, Lan, Liu, The essential role of mitochondrial dynamics in viral infections, Int. J. Mol. Sci, doi:10.3390/ijms26051955
Dunn, Connor, Hijakt, The PI3K/Akt pathway in virus replication and pathogenesis, Prog. Mol. Biol. Transl. Sci, doi:10.1016/B978-0-12-396456-4.00002-X
Dymkowska, Polonikov, Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00288
El Khoury, Naim, Lipid rafts disruption by statins negatively impacts the interaction between SARS-CoV-2 S1 subunit and ACE2 in intestinal epithelial cells, Front. Microbiol, doi:10.3389/fmicb.2023.1335458
Ergashev, Shi, Liu, Pan, Xie et al., a novel PFKFB3 inhibitor, prevent the progression of severe acute pancreatitis via the Nrf2/HO-1 pathway in infiltrated macrophage, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2023.11.010
Feingold, Anawalt, Blackman, Boyce, Chrousos et al., None
Feingold, Introduction to Lipids and Lipoproteins
Fernandes, De Brito, Reis, Sato, Pereira, SARS-CoV-2 and other respiratory viruses: What does oxidative stress have to do with it? Oxid, Med. Cell. Longev, doi:10.1155/2020/8844280
Ferrari, Rubini, Burns, The potential of purinergic signaling to thwart viruses including SARS-CoV-2, Front. Immunol, doi:10.3389/fimmu.2022.904419
Ferreira, Souza, Raposo, Ferreira, Silva, Xylitol inhibits J774A.1 macrophage adhesion in vitro, Braz. Arch. Biol. Technol, doi:10.1590/S1516-89132011000600017
Firpo, Mastrodomenico, Hawkins, Prot, Levillayer et al., Targeting polyamines inhibits coronavirus infection by reducing cellular attachment and entry, ACS Infect. Dis, doi:10.1021/acsinfecdis.0c00491
Fleming, Kolokoltsov, Davey, Nichols, Roberts et al., Respiratory syncytial virus F envelope protein associates with lipid rafts without a requirement for other virus proteins, J. Virol, doi:10.1128/JVI.00643-06
Florêncio De Mesquita, Rivera, Araújo, Durães, Queiroz et al., Adjunctive statin therapy in patients with Covid-19: A systematic review and meta-analysis of randomized controlled trials, Am. J. Med, doi:10.1016/j.amjmed.2024.06.002
Forrester, Kikuchi, Hernandes, Xu, Griendling, Reactive oxygen species in metabolic and inflammatory signaling, Circ. Res, doi:10.1161/CIRCRESAHA.117.311401
Fraser, Slessarev, Martin, Daley, Patel et al., Metabolomics profiling of critically ill coronavirus disease 2019 patients: Identification of diagnostic and prognostic biomarkers, Crit. Care Explor, doi:10.1097/CCE.0000000000000272
Fratta Pasini, Stranieri, Girelli, Busti, Cominacini, Is ferroptosis a key component of the process leading to multiorgan damage in COVID-19?, Antioxidants, doi:10.3390/antiox10111677
Fuertes-Martin, Moncayo, Insenser, Martínez-García, Luque-Ramírez et al., Glycoprotein A and B height-to-width ratios as obesity-independent novel biomarkers of low-grade chronic inflammation in women with polycystic ovary syndrome (PCOS), J. Proteome Res, doi:10.1021/acs.jproteome.9b00528
Fuertes-Martín, Taverner, Vallvé, Paredes, Masana et al., Characterization of 1H NMR plasma glycoproteins as a new strategy to identify inflammatory patterns in rheumatoid arthritis, J. Proteome Res, doi:10.1021/acs.jproteome.8b00411
Fujioka, Tsuda, Hattori, Sasaki, Sasaki et al., The Ras-PI3K signaling pathway is involved in clathrin-independent endocytosis and the internalization of influenza viruses, PLoS ONE, doi:10.1371/journal.pone.0016324
Féral, Jaud, Philippe, Di Bella, Pyronnet et al., ER stress and unfolded protein response in leukemia: Friend, Foe, or Both?, Biomolecules, doi:10.3390/biom11020199
Gabaldó, Juanpere, Castañé, Rodríguez-Tomàs, López-Azcona et al., Usefulness of the measurement of serum paraoxonase-1 arylesterase activity in the diagnoses of COVID-19, Biomolecules, doi:10.3390/biom12070879
Gasanoff, Yaguzhinsky, Garab, Cardiolipin, non-bilayer structures and mitochondrial bioenergetics: Relevance to cardiovascular disease, Cells, doi:10.3390/cells10071721
Gay, Desquiret-Dumas, Nagot, Rapenne, Van De Perre et al., Long-term persistence of mitochondrial dysfunctions after viral infections and antiviral therapies: A review of mechanisms involved, J. Med. Virol, doi:10.3390/life11030232
Geiler, Michaelis, Naczk, Leutz, Langer et al., N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus, Biochem. Pharmacol, doi:10.1016/j.bcp.2009.08.025
Ghini, Meoni, Vignoli, Di Cesare, Tenori et al., Fingerprinting and profiling in metabolomics of biosamples, Prog. Nucl. Magn. Reson. Spectrosc, doi:10.1016/j.pnmrs.2023.10.002
Ghini, Pecchioli, Celli, Boccia, Bertini et al., Metabolomic and lipoproteomic differences and similarities between COVID-19 and other types of pneumonia, Sci. Rep, doi:10.1038/s41598-025-91965-2
Girdhar, Guo, Regulating phase transition in neurodegenerative diseases by nuclear import rReceptors, Biology, doi:10.3390/biology11071009
Glende, Schwegmann-Wessels, Al-Falah, Pfefferle, Qu et al., Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2, Virology, doi:10.1016/j.virol.2008.08.026
Gouédard, Barouki, Morel, Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism, Mol. Cell. Biol, doi:10.1128/MCB.24.12.5209-5222.2004
Gravenstein, Foreword, Prevention of COVID-19, influenza, and respiratory syncytial virus in at-risk populations, Infect. Dis. Ther, doi:10.1007/s40121-024-01078-y
Greene, Choi, Chen, Yang, Li et al., Inhibiting glutamine metabolism blocks coronavirus replication in mammalian cells, bioRxiv, doi:10.1101/2023.09.27.559756
Greene, Choi, Yang, Chen, Li et al., Glutamine metabolism is essential for coronavirus replication in host cells and in mice, J. Biol. Chem, doi:10.1016/j.jbc.2024.108063
Gregorczyk-Zboroch, Szulc-D Ąbrowska, Pruchniak, Giery Ńska, Mielcarska et al., Modifications of mitochondrial network morphology affect the MAVS-dependent immune response in L929 murine fibroblasts during Ectromelia virus infection, Pathogens, doi:10.3390/pathogens13090717
Grimes, Khan, Badeaux, Rao, Rowlinson et al., Arginine depletion as a therapeutic approach for patients with COVID-19, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.10.100
Guarnieri, Dybas, Fazelinia, Kim, Frere et al., Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts, Sci. Transl. Med, doi:10.1126/scitranslmed.abq1533
Guarnieri, Lie, Albrecht, Hewin, Jurado et al., Mitochondrial antioxidants abate SARS-COV-2 pathology in mice, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2321972121
Guillemin, Brew, Implications of the kynurenine pathway and quinolinic acid in Alzheimer's disease, Redox Rep, doi:10.1179/135100002125000550
Guo, Wu, Li, Lin, Liu et al., Accelerated metabolite levels of aerobic glycolysis and the pentose phosphate pathway are required for efficient replication of infectious spleen and kidney necrosis virus in Chinese perch brain cells, Biomolecules, doi:10.3390/biom9090440
Guo, Zhu, Zhang, Meng, Zhu et al., Nuclear translocation of HIF-1α induced by influenza A (H1N1) infection is critical to the production of proinflammatory cytokines, Emerg. Microbes Infect, doi:10.1038/emi.2017.21
Gutierrez-Mariscal, Arenas-De Larriva, Limia-Perez, Romero-Cabrera, Yubero-Serrano et al., Coenzyme Q 10 supplementation for the reduction of oxidative stress: Clinical implications in the treatment of chronic diseases, Int. J. Mol. Sci, doi:10.3390/ijms21217870
Gutierrez-Mariscal, Yubero-Serrano, Villalba, Lopez-Miranda, Coenzyme Q 10 : From bench to clinic in aging diseases, a translational review, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2018.1442316
Gómez-Delgado, López-Pastor, González-Jiménez, Ramos-Acosta, Hernández-Garate et al., Long-term mitochondrial and metabolic impairment in lymphocytes of subjects who recovered after severe COVID-19, Cell Biol. Toxicol, doi:10.1007/s10565-024-09976-0
Hanage, Schaffner, Burden of acute respiratory infections caused by influenza virus, respiratory syncytial virus, and SARS-CoV-2 with consideration of older adults: A narrative review, Infect. Dis. Ther, doi:10.1007/s40121-024-01080-4
Hao, Zhang, Feng, Chen, Wan et al., Distinct lipid metabolic dysregulation in asymptomatic COVID-19, iScience, doi:10.1016/j.isci.2021.102974
Hardie, Ross, Hawley, AMPK: A nutrient and energy sensor that maintains energy homeostasis, Nat. Rev. Mol. Cell Biol, doi:10.1038/nrm3311
Harrison, Viral membrane fusion, Virology, doi:10.1016/j.virol.2015.03.043
Hasan Anber, Oied Saleh, Hassan Majed, Assessment of oxidative stress parameters in Iraqi male patients with Covid-19; A case control study, Rep. Biochem. Mol. Biol, doi:10.61186/rbmb.13.2.167
Hasin, Seldin, Lusis, Multi-omics approaches to disease, Genome Biol, doi:10.1186/s13059-017-1215-1
Havers, Whitaker, Melgar, Chatwani, Chai et al., Characteristics and outcomes among adults aged ≥60 years hospitalized with laboratory-confirmed respiratory syncytial virus-RSV-NET, 12 States, July 2022, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm7240a1
He, Zhang, Jiang, Zhu, Liang et al., PKM2 is a key factor to regulate neurogenesis and cognition by controlling lactate homeostasis, Stem Cell Rep, doi:10.1016/j.stemcr.2024.11.011
Heida, Bhide, Gasbarri, Kocabiyik, Stellacci et al., Advances in the development of entry inhibitors for sialic-acid-targeting viruses, Drug. Discov. Today, doi:10.1016/j.drudis.2020.10.009
Heinzl, Freudenthaler, Fellinger, Kolenchery, Resl et al., High-density lipoprotein predicts intrahospital mortality in influenza, J. Clin. Med, doi:10.3390/jcm13237242
Herrera-Moro Huitron, De Jesús-González, Martínez-Castillo, Ulloa-Aguilar, Cabello-Gutierrez et al., Multifaceted nature of lipid droplets in viral interactions and pathogenesis, Microorganisms, doi:10.3390/microorganisms11071851
Hima Bindu, Rao, Kakkar, Friend turns foe: Transformation of anti-inflammatory HDL to proinflammatory HDL during acute-phase response, Cholesterol, doi:10.1155/2011/274629
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hong, Boiti, Vallone, Foulkes, Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution, Antioxidants, doi:10.3390/antiox13030312
Hosakote, Liu, Castro, Garofalo, Casola, Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes, Am. J. Respir. Cell Mol. Biol, doi:10.1089/ars.2011.3999
Hsieh, Looney, Figueroa, Massaccesi, Stavrakis et al., Bystander monocytic cells drive infection-independent NLRP3 inflammasome response to SARS-CoV-2, mBio, doi:10.1128/mbio.00810-24
Hu, Liu, Zhang, Wang, Zhang et al., Functional metabolomics decipher biochemical functions and associated mechanisms underlie small-molecule metabolism, Mass Spectrom. Rev, doi:10.1002/mas.21611
Hu, Schulze, Ghildyal, Henstridge, Kolanowski et al., Respiratory syncytial virus co-opts host mitochondrial function to favour infectious virus production, Elife, doi:10.7554/eLife.42448
Huai, Mao, Wang, Lin, Li et al., How do RNA binding proteins trigger liquid-liquid phase separation in human health and diseases?, Biosci. Trends, doi:10.5582/bst.2022.01449
Huang, Chavda, Vora, Gajjar, Apostolopoulos et al., 2-deoxy-D-glucose and its derivatives for the COVID-19 treatment: An update, Front. Pharmacol, doi:10.3389/fphar.2022.899633
Huang, Jiang, Liu, Tang, Gui et al., Melatonin suppresses TLR4mediated RSV infection in the central nervous cells by inhibiting NLRP3 inflammasome formation and autophagy, J. Cell. Mol. Med, doi:10.1111/jcmm.18338
Ibrahim, Perl, Smith, Lewis, Kon et al., Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine, Clin. Immunol, doi:10.1016/j.clim.2020.108544
Iftimie, Amigó, Martínez-Micaelo, López-Azcona, Martínez-Navidad et al., Differential analysis of lipoprotein and glycoprotein profiles in bacterial infections and COVID-19 using proton nuclear magnetic resonance and machine learning, Heliyon, doi:10.1016/j.heliyon.2024.e37115
Iftimie, Gabaldó-Barrios, Penadés-Nadal, Canela-Capdevila, Piñana et al., Serum levels of arachidonic acid, interleukin-6, and C-reactive protein as potential indicators of pulmonary viral infections: Comparative analysis of influenza A, respiratory syncytial virus infection, and COVID-19, Viruses, doi:10.3390/v16071065
Igelmann, Lessard, Ferbeyre, Liquid-liquid phase separation in cancer signaling, metabolism and anticancer therapy, Cancers, doi:10.3390/cancers14071830
Isaacs, Kim, Thormar, Inactivation of enveloped viruses in human bodily fluids by purified lipids, Ann. N. Y. Acad. Sci, doi:10.1111/j.1749-6632.1994.tb38947.x
Ivanisevic, Want, From samples to insights into metabolism: Uncovering biologically relevant information in LC-HRMS metabolomics data, Meta, doi:10.3390/metabo9120308
Jantz-Naeem, Guvencli, Böttcher-Loschinski, Böttcher, Mougiakakos et al., Metabolic T-cell phenotypes: From bioenergetics to function, Am. J. Physiol. Cell Physiol, doi:10.1152/ajpcell.00478.2024
Jiang, Liu, Shen, Guo, Huang et al., Methyl-β-cyclodextrin inhibits EV-D68 virus entry by perturbing the accumulation of virus particles and ICAM-5 in lipid rafts, Antivir. Res, doi:10.1016/j.antiviral.2020.104752
Jin, Du, Xu, Deng, Liu et al., Structure of M pro from SARS-CoV-2 and discovery of its inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Jones, Qian, Van Der Velden, Chia, Mcmillan et al., Glutathione S-transferase pi modulates NF-κB activation and pro-inflammatory responses in lung epithelial cells, Redox Biol, doi:10.1016/j.redox.2016.03.005
Julkunen, Cicho Ńska, Slagboom, Würtz, Health et al., Metabolic biomarker profiling for identification of susceptibility to severe pneumonia and COVID-19 in the general population, Elife, doi:10.7554/eLife.63033
Kahan, Wherry, Zajac, T cell exhaustion during persistent viral infections, Virology, doi:10.1016/j.virol.2014.12.033
Kakad, Khopkar-Kale, Tripathy, Bhawalkar, Potential of melatonin as a treatment option for long COVID: A call for research, Br. J. Clin. Pharmacol, doi:10.1111/bcp.16375
Kaksonen, Roux, Mechanisms of clathrin-mediated endocytosis, Nat. Rev. Mol. Cell Biol, doi:10.1038/nrm.2017.132
Karimi, Chenari, Rezaie, Karimi, Parhizgari et al., Proposed pathway linking respiratory infections with depression, Clin. Psychopharmacol. Neurosci, doi:10.9758/cpn.2022.20.2.199
Keshavarz, Solaymani-Mohammadi, Namdari, Arjeini, Mousavi et al., Metabolic host response and therapeutic approaches to influenza infection, Cell. Mol. Biol. Lett, doi:10.1186/s11658-020-00211-2
Khaperskyy, Emara, Johnston, Anderson, Hatchette et al., Influenza A virus host shutoff disables antiviral stress-induced translation arrest, PLoS Pathog, doi:10.1371/journal.ppat.1004217
Khomich, Kochetkov, Bartosch, Ivanov, Redox biology of respiratory viral infections, Viruses, doi:10.3390/v10080392
Klarer, O'neal, Imbert-Fernandez, Clem, Ellis et al., Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism, Cancer Metab, doi:10.1186/2049-3002-2-2
Kleinehr, Wilden, Boergeling, Ludwig, Hrincius, Metabolic modifications by common respiratory viruses and their potential as new antiviral targets, Viruses, doi:10.3390/v13102068
Kolattukudy, Niu, Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway, Circ. Res, doi:10.1161/CIRCRESAHA.111.243212
Konaklieva, Plotkin, Targeting host-specific metabolic pathways-opportunities and challenges for anti-infective therapy, Front. Mol. Biosci, doi:10.3389/fmolb.2024.1338567
Kovtun, Tillu, Ariotti, Parton, Collins, Cavin family proteins and the assembly of caveolae, J. Cell Sci, doi:10.1242/jcs.167866
Kow, Hasan, Meta-analysis of effect of statins in patients with COVID-19, Am. J. Cardiol, doi:10.1016/j.amjcard.2020.08.004
Kumar, Sakharam, Tackling Influenza A virus by M2 ion channel blockers: Latest progress and limitations, doi:10.1016/j.ejmech.2024.116172
Kuss-Duerkop, Wang, Mena, White, Metreveli et al., Influenza virus differentially activates mTORC1 and mTORC2 signaling to maximize late stage replication, PLoS Pathog, doi:10.1371/journal.ppat.1006635
Kyle, Burnum-Johnson, Wendler, Eisfeld, Halfmann et al., Plasma lipidome reveals critical illness and recovery from human Ebola virus disease, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1815356116
Kyo, Zhu, Shibata, Fujiogi, Mansbach et al., Respiratory virus-specific nasopharyngeal lipidome signatures and severity in infants with bronchiolitis: A prospective multicenter study, J. Infect. Dis, doi:10.1093/infdis/jiad156
Lam, Zhang, Wang, Ni, Zhang et al., A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19, Nat. Metab, doi:10.1038/s42255-021-00425-4
Laplante, Sabatini, mTOR signaling in growth control and disease, Cell, doi:10.1016/j.cell.2012.03.017
Lara, Jr, Villanueva, Ibanez, Erman et al., A randomized, open-label, phase 3 trial of pembrolizumab plus epacadostat versus sunitinib or pazopanib as first-line treatment for metastatic renal cell carcinoma (KEYNOTE-679/ECHO-302), BMC Cancer, doi:10.1186/s12885-023-10971-7
Lee, Banoei, Ansari, Cheng, Lamontagne et al., Using a targeted metabolomics approach to explore differences in ARDS associated with COVID-19 compared to ARDS caused by H1N1 influenza and bacterial pneumonia, Crit. Care, doi:10.1186/s13054-024-04843-0
Lee, Shin, Zika virus modulates mitochondrial dynamics, mitophagy, and mitochondria-derived vesicles to facilitate viral replication in trophoblast cells, Front. Immunol, doi:10.3389/fimmu.2023.1203645
Li, Chen, Yang, Chiu, Modulating cholesterol-rich lipid rafts to disrupt influenza A virus infection, Front. Immunol, doi:10.3389/fimmu.2022.982264
Li, Ernst, Kolonko-Adamska, Greb-Markiewicz, Man et al., Phase separation in viral infections, Trends Microbiol, doi:10.1016/j.tim.2022.06.005
Li, Hou, Ma, Wang, Wang et al., SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy, Cell. Mol. Immunol, doi:10.1038/s41423-021-00807-4
Li, Ma, Fu, The mitochondrion-targeted antioxidants in kidney disease, Curr. Med. Chem, doi:10.2174/0929867327666201020151124
Li, Zhang, Gao, Wang, Zhang, 2 ′ -and 3 ′ -ribose modifications of nucleotide analogues establish the structural basis to inhibit the viral replication of SARS-CoV-2, J. Phys. Chem. Lett, doi:10.1021/acs.jpclett.2c00087
Liao, Li, Lei, Yu, Deng et al., Toxic effects of copper on the jejunum and colon of pigs: Mechanisms related to gut barrier dysfunction and inflammation influenced by the gut microbiota, Food Funct, doi:10.1039/D1FO01286J
Limsuwat, Boonarkart, Phakaratsakul, Suptawiwat, Auewarakul, Influence of cellular lipid content on influenza A virus replication, Arch. Virol, doi:10.1007/s00705-020-04596-5
Lin, Xue, Lu, Liu, Jiang et al., Multi-omics driven biomarker discovery and pathological insights into Pseudomonas aeruginosa pneumonia, BMC Infect. Dis, doi:10.1186/s12879-025-11119-7
Lionetto, Ulivieri, Capi, De Bernardini, Fazio et al., Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study, Biochim. Biophys. Acta Mol. Basis. Dis, doi:10.1016/j.bbadis.2020.166042
Liu, Le, Chen, Zhu, Lu, Role of PKM2-mediated immunometabolic reprogramming on development of cytokine storm, Front. Immunol, doi:10.3389/fimmu.2021.748573
Liu, Ma, Xu, Jin, Zhao et al., Adjunctive probiotics alleviates asthmatic symptoms via modulating the gut microbiome and serum metabolome, Microbiol. Spectr, doi:10.1128/Spectrum.00859-21
Liu, Wang, Wang, Guo, Song et al., Energy metabolism in health and diseases, Signal Transduct. Target. Ther, doi:10.1038/s41392-025-02141-x
Lodge, Nitschke, Kimhofer, Coudert, Begum et al., NMR spectroscopic windows on the systemic effects of SARS-CoV-2 infection on plasma lipoproteins and metabolites in relation to circulating cytokines, J. Proteome Res, doi:10.1021/acs.jproteome.0c00876
Longo, Frigeni, Pasquali, Carnitine transport and fatty acid oxidation, Biochim. Biophys. Acta, doi:10.1016/j.bbamcr.2016.01.023
Loo, Lodge, Kimhofer, Bong, Begum et al., Quantitative in-vitro diagnostic NMR spectroscopy for lipoprotein and metabolite measurements in plasma and serum: Recommendations for analytical artifact minimization with special reference to COVID-19/SARS-CoV-2 samples, J. Proteome Res, doi:10.1021/acs.jproteome.0c00537
Looft, Allen, Collateral effects of antibiotics on mammalian gut microbiomes, Gut Microbes, doi:10.4161/gmic.21288
Lorizate, Kräusslich, Role of lipids in virus replication, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a004820
Lu, Liu, Tam, Lipid rafts are involved in SARS-CoV entry into Vero E6 cells, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2008.02.023
Lu, Liu, Zhou, Liu, Yuan et al., Subgingival microbiome in periodontitis and type 2 diabetes mellitus: An exploratory study using metagenomic sequencing, J. Periodontal Implant Sci, doi:10.5051/jpis.2103460173
Lu, Xu, Sun, Shan, Shen et al., Analysis of temporal metabolic rewiring for human respiratory syncytial virus infection by integrating metabolomics and proteomics, Metabolomics, doi:10.1007/s11306-023-01991-2
Lu, Ye, Singh, Cao, Diedrich et al., The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein, Nat. Commun, doi:10.1038/s41467-020-20768-y
Luganini, Boschi, Lolli, Gribaudo, DHODH inhibitors: What will it take to get them into the clinic as antivirals?, Antivir. Res, doi:10.1016/j.antiviral.2025.106099
Låg, Skuland, Ballangby, Grytting, Jørgensen et al., Mechanisms involved in pro-inflammatory responses to traffic-derived particulate matter (PM) in THP-1 macrophages compared to HBEC3-KT bronchial epithelial cells, Toxicology, doi:10.1016/j.tox.2025.154174
López-Hernández, Oropeza-Valdez, García Lopez, Borrego, Murgu et al., Untargeted analysis in post-COVID-19 patients reveals dysregulated lipid pathways two years after recovery, Front. Mol. Biosci, doi:10.3389/fmolb.2023.1100486
Magulick, Frei, Ali, Mortensen, Pugh et al., The effect of statin therapy on the incidence of infections: A retrospective cohort analysis, Am. J. Med. Sci, doi:10.1097/MAJ.0b013e31828318e2
Mai, Tönjes, Kovacs, Stumvoll, Fiedler et al., Serum levels of acylcarnitines are altered in prediabetic conditions, PLoS ONE, doi:10.1371/journal.pone.0082459
Maio, Cherry, Schultz, Hurst, Linehan et al., TEMPOL inhibits SARS-CoV-2 replication and development of lung disease in the Syrian hamster model, iScience, doi:10.1016/j.isci.2022.105074
Majeed, Batool, Majeed, Bhat, Zargar et al., mTORC1 induces eukaryotic translation initiation factor 4E interaction with TOS-S6 kinase 1 and its activation, Cell Cycle, doi:10.1080/15384101.2021.1901038
Makowski, Chaib, Rathmell, Immunometabolism: From basic mechanisms to translation, Immunol. Rev, doi:10.1111/imr.12858
Mallol, Amigó, Rodríguez, Heras, Vinaixa et al., Liposcale: A novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy, J. Lipid Res, doi:10.1194/jlr.D050120
Mankouri, Tedbury, Gretton, Hughes, Griffin et al., Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0912426107
Marjuki, Gornitzky, Marathe, Ilyushina, Aldridge et al., Influenza A virus-induced early activation of ERK and PI3K mediates V-ATPase-dependent intracellular pH change required for fusion, Cell. Microbiol, doi:10.1111/j.1462-5822.2010.01556.x
Marsh, Helenius, Virus entry: Open sesame, Cell, doi:10.1016/j.cell.2006.02.007
Martín-Vicente, González-Riaño, Barbas, Jiménez-Sousa, Brochado-Kith et al., Metabolic changes during respiratory syncytial virus infection of epithelial cells, PLoS ONE, doi:10.1371/journal.pone.0230844
Mayneris-Perxachs, Moreno-Navarrete, Ballanti, Monteleone, Alessandro Paoluzi et al., Lipidomics and metabolomics signatures of SARS-CoV-2 mediators/receptors in peripheral leukocytes, jejunum and colon, Comput. Struct. Biotechnol. J, doi:10.1016/j.csbj.2021.11.007
Mayor, Parton, Donaldson, Clathrin-independent pathways of endocytosis, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a016758
Mazzarino, Targeting future pandemics, a case for de novo purine synthesis and basic research, Front. Immunol, doi:10.3389/fimmu.2021.694300
Mazzon, Mercer, Lipid interactions during virus entry and infection, Cell. Microbiol, doi:10.1111/cmi.12340
Mcmahon, Zajicek, Li, Peyton, Minna et al., SRBC/cavin-3 is a caveolin adapter protein that regulates caveolae function, EMBO J, doi:10.1038/emboj.2009.46
Mehmood, Nasir, Younis, Muanprasat, Inhibition of mitochondrial dynamics by mitochondrial division inhibitor-1 suppresses cell migration and metastatic markers in colorectal cancer HCT116 cells, J. Exp. Pharmacol, doi:10.2147/JEP.S510578
Mellor, Munn, IDO expression by dendritic cells: Tolerance and tryptophan catabolism, Nat. Rev. Immunol, doi:10.1038/nri1457
Meng, Zhu, Yang, Zhang, Jin et al., HIF-1α promotes virus replication and cytokine storm in H1N1 virus-induced severe pneumonia through cellular metabolic reprogramming, Virol. Sin, doi:10.1016/j.virs.2023.11.010
Meoni, Ghini, Maggi, Vignoli, Mazzoni et al., Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab, PLoS Pathog, doi:10.1371/journal.ppat.1009243
Meoni, Lorini, Monti, Madia, Corti et al., The metabolic fingerprints of HCV and HBV infections studied by Nuclear Magnetic Resonance Spectroscopy, Sci. Rep, doi:10.1038/s41598-019-40028-4
Miller, Silverstein, Flores, Cao, Kumagai et al., Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples, Sci. Rep, doi:10.1038/s41598-020-79552-z
Monks, Orlicky, Libby, Dzieciatkowska, Ladinsky et al., Perilipin-2 promotes lipid dropletplasma membrane interactions that facilitate apocrine lipid secretion in secretory epithelial cells of the mouse mammary gland, Front. Cell Dev. Biol, doi:10.3389/fcell.2022.958566
Moraes, Arginase and respiratory viral infections, Open Nitric Oxide J, doi:10.2174/1875042701002020064
Morris, Bortolasci, Puri, Olive, Marx et al., The pathophysiology of SARS-CoV-2: A suggested model and therapeutic approach, Life Sci, doi:10.1016/j.lfs.2020.118166
Mounce, Olsen, Vignuzzi, Connor, Polyamines and their role in virus infection, Microbiol. Mol. Biol. Rev, doi:10.1128/MMBR.00029-17
Nagata, Takeuchi, Masuoka, Aoki, Ishikane et al., Human gut microbiota and its metabolites impact immune responses in COVID-19 and its complications, Gastroenterology, doi:10.1053/j.gastro.2022.09.024
Naiditch, Betts, Larman, Levi, Rosenberg, Immunologic and inflammatory consequences of SARS-CoV-2 infection and its implications in renal disease, Front. Immunol, doi:10.3389/fimmu.2024.1376654
Naidu, Wang, Rao, Mancini, Clemens et al., Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long-COVID, NPJ Sci. Food, doi:10.1038/s41538-024-00261-2
Navab, Reddy, Van Lenten, Fogelman, HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms, Nat. Rev. Cardiol, doi:10.1038/nrcardio.2010.222
Nefedova, Koptev, Bobikova, Cherepushkina, Mironova et al., The infectious bronchitis coronavirus pneumonia model presenting a novel insight for the SARS-CoV-2 dissemination route, Vet. Sci, doi:10.3390/vetsci8100239
Nguyen, Bourredjem, Piroth, Bouhemad, Jalil et al., High plasma concentration of non-esterified polyunsaturated fatty acids is a specific feature of severe COVID-19 pneumonia, Sci. Rep, doi:10.1038/s41598-021-90362-9
Nichols, Nilsson, Brown-Harding, Laconte, Acker et al., Flexibility of the rotavirus NSP2 C-terminal region supports factory formation via liquid-liquid phase separation, J. Virol, doi:10.1128/jvi.00039-23
Nikolskiy, Siuzdak, Patti, Discriminating precursors of common fragments for large-scale metabolite profiling by triple quadrupole mass spectrometry, Bioinformatics, doi:10.1093/bioinformatics/btv085
O'flynn, Mittag, The role of liquid-liquid phase separation in regulating enzyme activity, Curr. Opin. Cell Biol, doi:10.1016/j.ceb.2020.12.012
Ohol, Wang, Kemble, Duke, Direct inhibition of cellular fatty acid synthase impairs replication of respiratory syncytial virus and other respiratory viruses, PLoS ONE, doi:10.1371/journal.pone.0144648
Okesli, Khosla, Bassik, Human pyrimidine nucleotide biosynthesis as a target for antiviral chemotherapy, Curr. Opin. Biotechnol, doi:10.1016/j.copbio.2017.03.010
Oliver, Reddy, Small molecules as therapeutic drugs for Alzheimer's disease, Mol. Cell. Neurosci, doi:10.1016/j.mcn.2019.03.001
Onorato, Pucci, Carpene, Henry, Sanchis-Gomar et al., Protective effects of statins administration in European and North American patients infected with COVID-19: A meta-analysis, Semin. Thromb. Hemost, doi:10.1055/s-0040-1722307
Otsubo, Bharathi, Uppala, Ilkayeva, Wang et al., Long-chain acylcarnitines reduce lung function by inhibiting pulmonary surfactant, J. Biol. Chem, doi:10.1074/jbc.M115.655837
Overmyer, Shishkova, Miller, Balnis, Bernstein et al., Large-scale multi-omic analysis of COVID-19 severity, Cell Syst, doi:10.1016/j.cels.2020.10.003
Pacheco-Hernández, Ramírez-Noyola, Gómez-García, Ignacio-Cortés, Zúñiga et al., Comparing the cytokine storms of COVID-19 and pandemic influenza, J. Interferon Cytokine Res, doi:10.1089/jir.2022.0029
Palacios-Rápalo, De Jesús-González, Cordero-Rivera, Farfan-Morales, Osuna-Ramos et al., Cholesterol-rich lipid rafts as platforms for SARS-CoV-2 entry, Front. Immunol, doi:10.3389/fimmu.2021.796855
Palmer, Innate metabolic responses against viral infections, Nat. Metab, doi:10.1038/s42255-022-00652-3
Pandey, Nour, Harris, Prominent receptors of liver sinusoidal endothelial cells in liver homeostasis and disease, Front. Physiol, doi:10.3389/fphys.2020.00873
Pandey, Zhou, Influenza A virus infection activates NLRP3 inflammasome through trans-Golgi network dispersion, Viruses, doi:10.3390/v14010088
Park, Liu, Liu, Zhou, Swine Influenza virus induces RIPK1/DRP1-mediated interleukin-1 beta production, Viruses, doi:10.3390/v10080419
Patti, Separation strategies for untargeted metabolomics, J. Sep. Sci, doi:10.1002/jssc.201100532
Pepe, Janes, Longton, Leisenring, Newcomb, Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker, Am. J. Epidemiol, doi:10.1093/aje/kwh101
Pike, Rafts defined: A report on the Keystone Symposium on Lipid Rafts and Cell Function, J. Lipid Res, doi:10.1194/jlr.E600002-JLR200
Pinero, Li, Liu, Li, Lee et al., Integrative multi-omics framework for causal gene discovery in long COVID, medRxiv, doi:10.1101/2025.02.09.25321751
Pouysségur, Marchiq, Parks, Durivault, Ždralević et al., Warburg effect' controls tumor growth, bacterial, viral infections and immunity-Genetic deconstruction and therapeutic perspectives, Semin. Cancer Biol, doi:10.1016/j.semcancer.2022.07.004
Purandare, Ghosalkar, Grossman, Aras, Mitochondrial oxidative phosphorylation in viral infections, Viruses, doi:10.3390/v15122380
Pérez, Gooz, Maldonado, Mitochondrial dysfunction and metabolic disturbances induced by viral infections, Cells, doi:10.3390/cells13211789
Qi, Yin, Xia, Yang, Exploring the role of mitochondrial antiviral signaling protein in cardiac diseases, Front. Immunol, doi:10.3389/fimmu.2025.1540774
Qin, Rao, Yuan, Wang, Zhao et al., SARS-CoV-2 couples evasion of inflammatory response to activated nucleotide synthesis, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2122897119
Qin, Wang, Han, Benefits of melatonin on mortality in severe-to-critical COVID-19 patients: A systematic review and meta-analysis of randomized controlled trials, Clinics, doi:10.1016/j.clinsp.2025.100638
Qu, Haas De Mello, Morris, Jones-Hall, Ivanciuc et al., SARS-CoV-2 inhibits NRF2-mediated antioxidant responses in airway epithelial cells and in the lung of a murine model of infection, Microbiol. Spectr, doi:10.1128/spectrum.00378-23
Rashid, Yadav, Kim, Chae, ER stress: Autophagy induction, inhibition and selection, Autophagy, doi:10.1080/15548627.2015.1091141
Rawat, Viard, Gallo, Rein, Blumenthal et al., Modulation of entry of enveloped viruses by cholesterol and sphingolipids, Mol. Membr. Biol, doi:10.1080/0968768031000104944
Relja, Land, Damage-associated molecular patterns in trauma, Eur. J. Trauma Emerg. Surg, doi:10.1007/s00068-019-01235-w
Ren, Zhang, Zhang, Zhang, Zhang et al., Influenza A virus (H1N1) infection induces glycolysis to facilitate viral replication, Virol. Sin, doi:10.1007/s12250-021-00433-4
Reyes, Duarte, Farías, Tognarelli, Kalergis et al., Impact of hypoxia over human viral infections and key cellular processes, Int. J. Mol. Sci, doi:10.3390/ijms22157954
Riera-Borrull, Rodríguez-Gallego, Hernández-Aguilera, Luciano, Ras et al., Exploring the process of energy generation in pathophysiology by targeted metabolomics: Performance of a simple and quantitative method, J. Am. Soc. Mass Spectrom, doi:10.1007/s13361-015-1262-3
Ripa, Andreu, López-Guerrero, Bello-Morales, Membrane rafts: Portals for viral entry, Front. Microbiol, doi:10.3389/fmicb.2021.631274
Risso-Ballester, Rameix-Welti, Spatial resolution of virus replication: RSV and cytoplasmic inclusion bodies, Adv. Virus Res, doi:10.1016/bs.aivir.2023.06.001
Robinson, Talty, Logue, Mnich, Gorman et al., An emerging role for the unfolded protein response in pancreatic cancer, Cancers, doi:10.3390/cancers13020261
Rocha, Hernandez-Mijares, Garcia-Malpartida, Bañuls, Bellod et al., Mitochondria-targeted antioxidant peptides, Curr. Pharm. Des, doi:10.2174/138161210793292519
Rodríguez-Tomàs, Iftimie, Castañé, Baiges-Gaya, Hernández-Aguilera et al., Clinical performance of paraoxonase-1-related variables and novel markers of inflammation in coronavirus disease-19. A machine learning approach, Antioxidants, doi:10.3390/antiox10060991
Rodríguez-Vera, Salazar, Soriano-Ursúa, Guzmán-Pérez, Vergara-Castañeda et al., Effectiveness of omega-3 fatty acid supplementation in improving the metabolic and inflammatory profiles of Mexican adults hospitalized with COVID-19, Diseases, doi:10.3390/diseases12010028
Roncato, Angelini, Pani, Talotta, Lipid rafts as viral entry routes and immune platforms: A double-edged sword in SARS-CoV-2 infection?, Biochim. Biophys. Acta Mol. Cell Biol. Lipids, doi:10.1016/j.bbalip.2022.159140
Rossman, Leser, Lamb, Filamentous influenza virus enters cells via macropinocytosis, J. Virol, doi:10.1128/JVI.05992-11
Rothhammer, Quintana, The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease, Nat. Rev. Immunol, doi:10.1038/s41577-019-0125-8
Russell, Gold, Willing, Thorson, Mcnagny et al., Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma, Gut Microbes, doi:10.4161/gmic.23567
Ruzzi, Cappello, Semprini, Scalambra, Angelicola et al., Lipid rafts, caveolae, and epidermal growth factor receptor family: Friends or foes?, Cell Commun. Signal, doi:10.1186/s12964-024-01876-4
Rössler, Berezhnoy, Singh, Cannet, Reinsperger et al., Quantitative serum NMR spectroscopy stratifies COVID-19 patients and sheds light on interfaces of host metabolism and the immune response with cytokines and clinical parameters, Metabolites, doi:10.3390/metabo12121277
Safaei Ardestani, Rahideh, The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial, J. Transl. Med, doi:10.1186/s12967-022-03237-6
Safiri, Mahmoodpoor, Kolahi, Nejadghaderi, Sullman et al., Global burden of lower respiratory infections during the last three decades, Front. Public Health, doi:10.3389/fpubh.2022.1028525
Sage, Cinti, Amorim, Mouland, Adapting the stress response: Viral subversion of the mTOR signaling pathway, Viruses, doi:10.3390/v8060152
Sahan, Hazra, Das, The pivotal role of DNA repair in infection mediated-inflammation and cancer, Front. Microbiol, doi:10.3389/fmicb.2018.00663
Saito, Kimura, Roles of phase separation for cellular redox maintenance, Front. Genet, doi:10.3389/fgene.2021.691946
Sanchez, Lagunoff, Viral activation of cellular metabolism, Virology, doi:10.1016/j.virol.2015.02.038
Sandepogu, Dara, Mallamgunta, Jogi, Sree Podila et al., Role of 2-deoxy-D-glucose in enhancing the efficacy of standard of care for moderate to severe COVID-19: A comparative analysis of clinical outcomes, Cureus, doi:10.7759/cureus.73993
Sandvig, Kavaliauskiene, Skotland, Clathrin-independent endocytosis: An increasing degree of complexity, Histochem. Cell Biol, doi:10.1007/s00418-018-1678-5
Santos, Póvoa, Paixão, Mendonça, Taborda-Barata, Changes in glycolytic pathway in SARS-COV 2 infection and their importance in understanding the severity of COVID-19, Front. Chem, doi:10.3389/fchem.2021.685196
Savastano, Ibáñez De Opakua, Rankovic, Zweckstetter, Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates, Nat. Commun, doi:10.1038/s41467-020-19843-1
Schmelter, Föh, Mallagaray, Rahmöller, Ehlers et al., Metabolic and lipidomic markers differentiate COVID-19 from non-hospitalized and other intensive care patients, Front. Mol. Biosci, doi:10.3389/fmolb.2021.737039
Sen, Kaminiski, Deshmane, Langford, Khalili et al., Role of hexokinase-1 in the survival of HIV-1-infected macrophages, Cell Cycle, doi:10.1080/15384101.2015.1006971
Shafaq-Zadah, Dransart, Johannes, Clathrin-independent endocytosis, retrograde trafficking, and cell polarity, Curr. Opin. Cell Biol, doi:10.1016/j.ceb.2020.05.009
Shahpar, Sofiani, Nezhad, Charostad, Ghaderi et al., A narrative review: Exploring viral-induced malignancies through the lens of dysregulated cellular metabolism and glucose transporters, BMC Cancer, doi:10.1186/s12885-024-13013-y
Shaikh, Crowe, Jr, Molecular mechanisms driving respiratory syncytial virus assembly, Future Microbiol, doi:10.2217/fmb.12.132
Shamilov, Ackley, Aneskievich, Enhanced wound healing -and inflammasome-associated gene expression in TNFAIP3-interacting protein 1-(TNIP1-) deficient HaCaT keratinocytes parallels reduced reepithelialization, Mediat. Inflamm, doi:10.1155/2020/5919150
Shang, Liu, Zhu, Lu, Ge et al., SARS-CoV-2 causes mitochondrial dysfunction and mitophagy impairment, Front. Microbiol, doi:10.3389/fmicb.2021.780768
Shen, Yi, Sun, Bi, Du et al., Proteomic and metabolomic characterization of COVID-19 patient sera, Cell, doi:10.1016/j.cell.2020.05.032
Shih, Erb, Sun, Toda, Kalivas et al., Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation, J. Neurosci, doi:10.1523/JNEUROSCI.3178-06.2006
Shikama, Otsuka, Shikama, Furukawa, Ishimaru et al., Involvement of metformin and aging in salivary expression of ACE2 and TMPRSS2, Biofactors, doi:10.1002/biof.2154
Shukla, Budden, Neal, Hansbro, Microbiome effects on immunity, health and disease in the lung, Clin. Transl. Immunol, doi:10.1038/cti.2017.6
Silva, Da Silva, Amaral, Fragas, Câmara, Metabolic alterations in SARS-CoV-2 infection and its implication in kidney dysfunction, Front. Physiol, doi:10.3389/fphys.2021.624698
Silvagno, Vernone, Pescarmona, The role of glutathione in protecting against the severe inflammatory response triggered by COVID-19, Antioxidants, doi:10.3390/antiox9070624
Simons, Sampaio, Membrane organization and lipid rafts, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a004697
Singh, Amar, Gehlot, Patra, Kanwar et al., Mitochondrial modulations, autophagy pathways shifts in viral infections: Consequences of COVID-19, Int. J. Mol. Sci, doi:10.3390/ijms22158180
Singh, Singh, Suhail, Arumugaswami, Pellett et al., AMP-activated protein kinase restricts Zika virus replication in endothelial cells by potentiating innate antiviral responses and inhibiting glycolysis, J. Immunol. Baltim. Md, doi:10.4049/jimmunol.1901310
Slaine, Kleer, Duguay, Pringle, Kadijk et al., Thiopurines activate an antiviral unfolded protein response that blocks influenza A virus glycoprotein accumulation, J. Virol, doi:10.1128/JVI.00453-21
Smallwood, Duan, Morfouace, Rezinciuc, Shulkin et al., Targeting metabolic reprogramming by influenza infection for therapeutic intervention, Cell Rep, doi:10.1016/j.celrep.2017.04.039
Song, Lam, Fan, Cao, Wang et al., Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis, Cell Metab, doi:10.1016/j.cmet.2020.06.016
Song, Lee, Lee, Park, Yang, Host subcellular organelles: Targets of viral manipulation, Int. J. Mol. Sci, doi:10.3390/ijms25031638
Song, Zhu, Qiu, Cai, Hu et al., A new mechanism of respiratory syncytial virus entry inhibition by small-molecule to overcome K394R-associated resistance, mBio, doi:10.1128/mbio.01385-24
Stefano, Weissenberger, Ptacek, Anders, Raboch et al., Viruses and mitochondrial dysfunction in neurodegeneration and cognition: An evolutionary perspective, Cell. Mol. Neurobiol, doi:10.1007/s10571-024-01503-3
Stincone, Prigione, Cramer, Wamelink, Campbell et al., The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway, Biol. Rev. Camb. Philos. Soc, doi:10.1111/brv.12140
Stone, Williams, Modulation of T cells by tryptophan metabolites in the kynurenine pathway, Trends Pharmacol. Sci, doi:10.1016/j.tips.2023.04.006
Strimbu, Tavel, What are biomarkers?, doi:10.1097/COH.0b013e32833ed177
Sun, Zhang, Song, Fang, Liu et al., Functional metabolomics discover pentose and glucuronate interconversion pathways as promising targets for Yang Huang syndrome treatment with Yinchenhao Tang, RSC Adv, doi:10.1039/C8RA06553E
Swanson, Deng, Ting, The NLRP3 inflammasome: Molecular activation and regulation to therapeutics, Nat. Rev. Immunol, doi:10.1038/s41577-019-0165-0
Talley, Mohiuddin, Biochemistry, Fatty Acid Oxidation
Tang, Zhang, Yang, Shi, Fu et al., TGEV NSP1 enhances viral replication through antagonizing stress granule formation, Vet. Microbiol, doi:10.1016/j.vetmic.2025.110502
Tanner, Chng, Guan, Lei, Rozen et al., Lipidomics identifies a requirement for peroxisomal function during influenza virus replication, J. Lipid Res, doi:10.1194/jlr.M049148
Tavassolifar, Aghdaei, Sadatpour, Maleknia, Fayazzadeh et al., New insights into extracellular and intracellular redox status in COVID-19 patients, Redox Biol, doi:10.1016/j.redox.2022.102563
Teer, Mukonowenzou, Essop, Hiv, inflammation, and immunometabolism: A model of the inflammatory theory of disease, Viruses, doi:10.3390/v17060839
Thaker, Ch'ng, Christofk, Viral hijacking of cellular metabolism, BMC Biol, doi:10.1186/s12915-019-0678-9
Thomas, Stefanoni, Reisz, Nemkov, Bertolone et al., COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status, JCI Insight, doi:10.1172/jci.insight.140327
Thormar, Isaacs, Brown, Barshatzky, Pessolano, Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides, Antimicrob. Agents Chemother
Tirkan, Eskandari, Roham, Aloosh, Ramim et al., Investigating the effectiveness of melatonin in the treatment of critically ill patients with COVID-19 hospitalized in the Intensive Care Unit: A double-blind randomized clinical trial, Med. J. Islam. Repub. Iran, doi:10.47176/mjiri.38.41
Tong, Hannou, Wang, Astapova, Sargsyan et al., The intestine is a major contributor to circulating succinate in mice, FASEB J, doi:10.1096/fj.202200135RR
Torrente-Rodríguez, Ruiz-Valdepeñas, Montiel, Iftimie, Montero-Calle et al., Contributing to the management of viral infections through simple immunosensing of the arachidonic acid serum level, Mikrochim. Acta, doi:10.1007/s00604-024-06440-y
Twu, Lee, Kim, Prasad, Cerikan et al., Contribution of autophagy machinery factors to HCV and SARS-CoV-2 replication organelle formation, Cell Rep, doi:10.1016/j.celrep.2021.110049
Tábara, Morris, Prudent, The complex dance of organelles during mitochondrial division, Trends Cell Biol, doi:10.1016/j.tcb.2020.12.005
Vahedian-Azimi, Mannarino, Shojaie, Rahimibashar, Galeh et al., The effect of statins on the prevalence and mortality of influenza virus infection: A systematic review and meta-analysis, Arch. Med. Sci, doi:10.5114/aoms/149633
Van Den Berg, Meurs, Gosens, Targeting arginase and nitric oxide metabolism in chronic airway diseases and their co-morbidities, Curr. Opin. Pharmacol, doi:10.1016/j.coph.2018.04.010
Van Lenten, Wagner, Anantharamaiah, Garber, Fishbein et al., Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide, Circulation, doi:10.1161/01.CIR.0000030182.35880.3E
Van Royen, Rossey, Sedeyn, Schepens, Saelens, How RSV proteins join forces to overcome the host innate immune response, Viruses, doi:10.3390/v14020419
Vanhaesebroeck, Stephens, Hawkins, PI3K signalling: The path to discovery and understanding, Nat. Rev. Mol. Cell Biol, doi:10.1038/nrm3290
Vazquez, Horner, MAVS coordination of antiviral innate immunity, J. Virol, doi:10.1128/JVI.01918-14
Verma, Adhikary, Woloschak, Dwarakanath, Papineni, A combinatorial approach of a polypharmacological adjuvant 2-deoxy-D-glucose with low dose radiation therapy to quell the cytokine storm in COVID-19 management, Int. J. Radiat. Biol, doi:10.1080/09553002.2020.1818865
Verma, Gupta, Lal, Host lipid rafts play a major role in binding and endocytosis of Influenza A virus, Viruses, doi:10.3390/v10110650
Vicinanza, Di Campli, De Matteis, Function and dysfunction of the PI system in membrane trafficking, EMBO J, doi:10.1038/emboj.2008.169
Vincent, Ziehr, Moorman, Human cytomegalovirus strategies to maintain and promote mRNA translation, Viruses, doi:10.3390/v8040097
Waheed, Freed, The role of lipids in retrovirus replication, Viruses, doi:10.3390/v2051146
Wan, Zhang, He, Tian, Lin et al., Gut microbiota and metabolite changes in patients with ulcerative colitis and Clostridioides difficile infection, Front. Microbiol, doi:10.3389/fmicb.2022.802823
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Khoramjoo, Srinivasan, Gordon, Mandal et al., Sequential multi-omics analysis identifies clinical phenotypes and predictive biomarkers for long COVID, Cell Rep. Med, doi:10.1016/j.xcrm.2023.101254
Wang, Zhang, Dai, Qin, Lu et al., Liquid-liquid phase separation in human health and diseases, Signal Transduct. Target. Ther, doi:10.1038/s41392-021-00678-1
Wang, Zhou, Phase separation as a new form of regulation in innate immunity, Mol. Cell, doi:10.1016/j.molcel.2024.06.004
Wang, Zhu, Ren, Yang, Tian et al., Influenza A virus protein PB1-F2 impairs innate immunity by inducing mitophagy, Autophagy, doi:10.1080/15548627.2020.1725375
Washirasaksiri, Sayabovorn, Ariyakunaphan, Kositamongkol, Chaisathaphol et al., Long-term multiple metabolic abnormalities among healthy and high-risk people following nonsevere COVID-19, Sci. Rep, doi:10.1038/s41598-023-41523-5
Watkins, Degrado, Voth, Influenza A M2 inhibitor binding understood through mechanisms of excess proton stabilization and channel dynamics, J. Am. Chem. Soc, doi:10.1021/jacs.0c06419
Wei, Ye, Zhang, Hu, Wu et al., Melatonin rescues Influenza A virus-induced cellular energy exhaustion via OMA1-OPA1-S in acute exacerbation of COPD, J. Pineal Res, doi:10.1111/jpi.12991
West, Merle, Kami Ński, Palacios, Kumar et al., Loss of CD4+ T cell-intrinsic arginase 1 accelerates Th1 response kinetics and reduces lung pathology during influenza infection, Immunity, doi:10.1016/j.immuni.2023.07.014
Westermann, Mitochondrial fusion and fission in cell life and death, Nat. Rev. Mol. Cell Biol, doi:10.1038/nrm3013
Williams, Jureka, Silvas, Nicolini, Chvatal et al., Inhibitors of VPS34 and fatty-acid metabolism suppress SARS-CoV-2 replication, Cell Rep, doi:10.1016/j.celrep.2021.109479
Wu, Li, Zeng, Qiao, Zhou, Serum metabolomics analysis of the anti-inflammatory effects of gallic acid on rats with acute inflammation, Front. Pharmacol, doi:10.3389/fphar.2022.830439
Wu, Meininger, Mcneal, Bazer, Rhoads, Role of L-arginine in nitric oxide synthesis and health in humans, Adv. Exp. Med. Biol, doi:10.1007/978-3-030-74180-8_10
Wu, Shu, Yang, Song, Zhang et al., Plasma metabolomic and lipidomic alterations associated with COVID-19, Natl. Sci. Rev, doi:10.1093/nsr/nwaa086
Wu, Yan, Jiang, Chen, Du et al., Metabolic regulation of dendritic cell activation and immune function during inflammation, Front. Immunol, doi:10.3389/fimmu.2023.1140749
Wölk, Fedorova, The lipid droplet lipidome, FEBS Lett, doi:10.1002/1873-3468.14874
Xie, Cho, Lin, Pillai, Heimisdottir et al., Improved metabolite prediction using microbiome data-based elastic net models, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2021.734416
Xie, Lei, Zhao, Guo, Cui, mTOR in programmed cell death and its therapeutic implications, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2023.06.002
Xiong, Li, Zhao, Li, Yu et al., Integrated serum pharmacochemistry, metabolomics, and network pharmacology to reveal the material basis and mechanism of Danggui Shaoyao San in the treatment of primary dysmenorrhea, Front. Pharmacol, doi:10.3389/fphar.2022.942955
Xu, Ilyas, Weng, Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies, Acta Pharmacol. Sin, doi:10.1038/s41401-022-00998-0
Xu, Kang, Wang, Zuo, Tan et al., Melatonin improves influenza virus infection-induced acute exacerbation of COPD by suppressing macrophage M1 polarization and apoptosis, Respir. Res, doi:10.1186/s12931-024-02815-0
Xu, Mak, Lukmantara, Li, Hoehn et al., CDP-DAG synthase 1 and 2 regulate lipid droplet growth through distinct mechanisms, J. Biol. Chem, doi:10.1074/jbc.RA119.009992
Xu, Wi, Kim, Kim, Ameliorating effect of dietary xylitol on human respiratory syncytial virus (hRSV) infection, Biol. Pharm. Bull, doi:10.1248/bpb.b15-00773
Xu, Zhao, Zhu, Wen, Li, Mitofusin-mediated mitochondrial fusion inhibits Pseudorabies virus infection in porcine cells, Vet. Sci, doi:10.3390/vetsci12040368
Yan, Chen, Liang, Zheng, Ye et al., Metabolomics profile in acute respiratory distress syndrome by nuclear magnetic resonance spectroscopy in patients with community-acquired pneumonia, Respir. Res, doi:10.1186/s12931-022-02075-w
Yan, Chu, Yang, Sze, Lai et al., Characterization of the lipidomic profile of human coronavirus-infected cells: Implications for lipid metabolism remodeling upon coronavirus replication, Viruses, doi:10.3390/v11010073
Yang, Liu, Nie, Zhan, Zhu, Oxidative stress and ROS-mediated cellular events in RSV infection: Potential protective roles of antioxidants, Virol. J, doi:10.1186/s12985-023-02194-w
Yang, Wang, Liu, Wang, Wu, TPM4 condensates glycolytic enzymes and facilitates actin reorganization under hyperosmotic stress, Cell Discov, doi:10.1038/s41421-024-00744-2
Yang, Willis, Button, Strang, Fu et al., Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response, Nat. Commun, doi:10.1038/s41467-019-11671-2
Yang, Yi, Kim, Kim, Park et al., Nuclear factor kappa-Band activator protein-1-mediated immunostimulatory activity of compound K in monocytes and macrophages, J. Ginseng Res, doi:10.1016/j.jgr.2016.06.004
Yen, Wei, Shih, Hsu, Hsu et al., Metformin use before Influenza vaccination may lower the risks of influenza and related complications, Vaccines, doi:10.3390/vaccines10101752
Yin, Kim, Kim, Protective effect of dietary xylitol on influenza A virus infection, PLoS ONE, doi:10.1371/journal.pone.0084633
Yin, Li, Han, Liu, Zeng et al., Long-term effects of lysine concentration on growth performance, intestinal microbiome, and metabolic profiles in a pig model, Food Funct, doi:10.1039/C8FO00973B
Yoo, Yu, Sung, Han, Glutamine reliance in cell metabolism, Exp. Mol. Med, doi:10.1038/s12276-020-00504-8
Yu, Shang, Guo, Zhang, Zhang et al., The gut microbiome in differential diagnosis of diabetic kidney disease and membranous nephropathy, Ren. Fail, doi:10.1080/0886022X.2020.1837869
Yuan, Ye, Chu, Host PFKFB3-dependent glycolytic reprogramming as a broad-spectrum antiviral strategy, Open Forum Infect. Dis, doi:10.1093/ofid/ofad500.976
Zachrdla, Savastano, Ibáñez De Opakua, Cima-Omori, Zweckstetter, Contributions of the N-terminal intrinsically disordered region of the severe acute respiratory syndrome coronavirus 2 nucleocapsid protein to RNA-induced phase separation, Protein Sci, doi:10.1002/pro.4409
Zhang, Wang, Ni, Di, Ma et al., COVID-19: Melatonin as a potential adjuvant treatment, Life Sci, doi:10.1016/j.lfs.2020.117583
Zhang, Whittaker, Influenza entry pathways in polarized MDCK cells, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2014.05.095
Zhao, Chen, Cheng, Xu, Yang et al., Deficiency of HIF-1α enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1742585
Ziehr, Macdonald, Regulation of NLRPs by reactive oxygen species: A story of crosstalk, Biochim. Biophys. Acta Mol. Cell Res, doi:10.1016/j.bbamcr.2024.119823
Zimodro, Mucha, Berthold, Gouni-Berthold, Lipoprotein metabolism, dyslipidemia, and lipid-lowering therapy in women: A comprehensive review, Pharmaceuticals, doi:10.3390/ph17070913
Zumla, Rao, Wallis, Kaufmann, Rustomjee et al., Host-directed therapies for infectious diseases: Current status, recent progress, and future prospects, Lancet Infect. Dis, doi:10.1016/S1473-3099(16)00078-5
DOI record:
{
"DOI": "10.3390/biom15071027",
"ISSN": [
"2218-273X"
],
"URL": "http://dx.doi.org/10.3390/biom15071027",
"abstract": "<jats:p>Respiratory infections caused by severe acute respiratory syndrome coronavirus 2, influenza virus, and respiratory syncytial virus pose significant global health challenges, leading to high morbidity and mortality, particularly in vulnerable populations. Despite their distinct virological characteristics, these viruses exploit host cellular metabolism to support replication, modulate immune responses, and promote disease progression. Emerging evidence shows that they induce metabolic reprogramming, shifting cellular energy production toward glycolysis to meet the bioenergetic demands of viral replication. Additionally, alterations in lipid metabolism, including enhanced fatty acid synthesis and disrupted cholesterol homeostasis, facilitate viral entry, replication, and immune evasion. The dysregulation of mitochondrial function and oxidative stress pathways also contributes to disease severity and long-term complications, such as persistent inflammation and immune exhaustion. Understanding these metabolic shifts is crucial for identifying new therapeutic targets and novel biomarkers for early disease detection, prognosis, and patient stratification. This review provides an overview of the metabolic alterations induced by severe acute respiratory syndrome coronavirus 2, influenza virus, and respiratory syncytial virus, highlighting shared and virus-specific mechanisms and potential therapeutic interventions.</jats:p>",
"alternative-id": [
"biom15071027"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-3165-3640",
"affiliation": [
{
"name": "Unitat de Recerca Biomèdica, Hospital Universitari de Sant Joan, Institut d’Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Av. Dr. Josep Laporte 2, 43204 Reus, Catalonia, Spain"
}
],
"authenticated-orcid": false,
"family": "Camps",
"given": "Jordi",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0003-0714-8414",
"affiliation": [
{
"name": "Autoimmunity, Infection and Thrombosis Research Group (GRAIIT), Department of Internal Medicine, Hospital Universitari de Sant Joan, Institut d’Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Av. Dr. Josep Laporte 2, 43204 Reus, Catalonia, Spain"
}
],
"authenticated-orcid": false,
"family": "Iftimie",
"given": "Simona",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4251-585X",
"affiliation": [
{
"name": "Unitat de Recerca Biomèdica, Hospital Universitari de Sant Joan, Institut d’Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Av. Dr. Josep Laporte 2, 43204 Reus, Catalonia, Spain"
}
],
"authenticated-orcid": false,
"family": "Jiménez-Franco",
"given": "Andrea",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5441-6333",
"affiliation": [
{
"name": "Autoimmunity, Infection and Thrombosis Research Group (GRAIIT), Department of Internal Medicine, Hospital Universitari de Sant Joan, Institut d’Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Av. Dr. Josep Laporte 2, 43204 Reus, Catalonia, Spain"
}
],
"authenticated-orcid": false,
"family": "Castro",
"given": "Antoni",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2749-4541",
"affiliation": [
{
"name": "Unitat de Recerca Biomèdica, Hospital Universitari de Sant Joan, Institut d’Investigació Sanitària Pere Virgili, Universitat Rovira i Virgili, Av. Dr. Josep Laporte 2, 43204 Reus, Catalonia, Spain"
}
],
"authenticated-orcid": false,
"family": "Joven",
"given": "Jorge",
"sequence": "additional"
}
],
"container-title": "Biomolecules",
"container-title-short": "Biomolecules",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
7,
16
]
],
"date-time": "2025-07-16T15:48:22Z",
"timestamp": 1752680902000
},
"deposited": {
"date-parts": [
[
2025,
7,
16
]
],
"date-time": "2025-07-16T16:04:57Z",
"timestamp": 1752681897000
},
"indexed": {
"date-parts": [
[
2025,
7,
16
]
],
"date-time": "2025-07-16T16:40:10Z",
"timestamp": 1752684010705,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2025,
7,
16
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2025,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
16
]
],
"date-time": "2025-07-16T00:00:00Z",
"timestamp": 1752624000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2218-273X/15/7/1027/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1027",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2025,
7,
16
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
16
]
]
},
"publisher": "MDPI AG",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2218-273X/15/7/1027"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Metabolic Reprogramming in Respiratory Viral Infections: A Focus on SARS-CoV-2, Influenza, and Respiratory Syncytial Virus",
"type": "journal-article",
"volume": "15"
}