Mitigating COVID-19 Mortality and Morbidity in China's Aging Population: A Focus on Available Medications and Future Developments
, E., Aging and disease, doi:10.14336/AD.2023.0318, Dec 2023
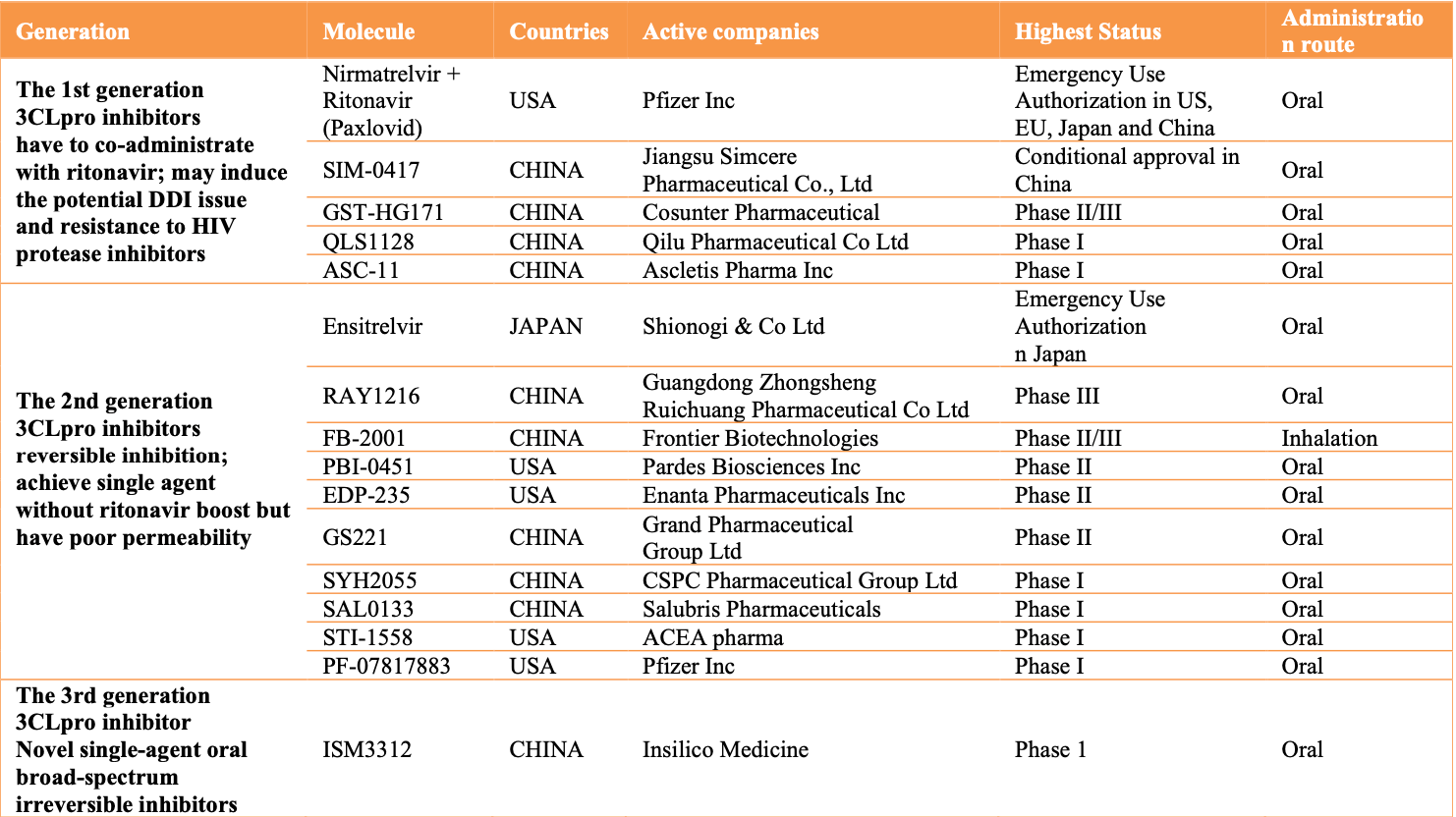
Review focusing on 3CL protease inhibitors. First generation inhibitors like paxlovid and simnotrelvir require boosting with ritonavir, which can cause drug-drug interactions and other issues. Second generation inhibitors like ensitrelvir achieve single agent use without ritonavir boosting, but may have bioavailability issues leading to need for higher doses. Third generation inhibitors aim to achieve irreversible single agent inhibition with higher oral bioavailability and lower viral resistance.
Review covers paxlovid, ensitrelvir, and xiannuoxin.
1.
Shen et al., Carboxylesterase Factors Influencing the Therapeutic Activity of Common Antiviral Medications Used for SARS-CoV-2 Infection, Pharmaceutics, doi:10.3390/pharmaceutics17070832.
2.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
3.
Brewitz et al., Fixing the Achilles Heel of Pfizer’s Paxlovid for COVID-19 Treatment, Journal of Medicinal Chemistry, doi:10.1021/acs.jmedchem.4c01342.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
Bischof et al., 31 Dec 2023, peer-reviewed, 1 author.
Contact: bischofevelyne@gmail.com.
Mitigating COVID-19 Mortality and Morbidity in China's Aging Population: A Focus on Available Medications and Future Developments
Aging and disease, doi:10.14336/ad.2023.0318
The COVID-19 pandemic, often referred to as the geropandemic, has put immense pressure on global healthcare systems worldwide, leading to a rush in the development and approval of medications for the treatment of the viral infection. Clinical trials on efficacy and safety had a limited spectrum on inclusion and endpoints because of the urgent need for fast results. The chronologically and biologically aged population is especially at risk for severe or lethal disease, as well as treatment-associated toxicity. In China, the growing elderly population segment has been a focus in public health measurements of COVID-19, guiding towards herd immunity with a mild variant, thus minimizing overall deaths and morbidity. While the COVID-19 pandemic has now been reclassified and the virus weakened, there is a clear need for novel therapies to protect the elderly. This paper reviews the current safety and efficacy of available COVID-19 medications in China, with a specific focus on 3CL protease inhibitors and the aging population. The current COVID wave in China has demonstrated a significant impact on the elderly and the need for new drugs that are effective at low doses and can be used alone, without harmful side effects, generation of viral resistance, and drug-drug interactions. The rush to develop and approve COVID-19 medications has brought up important questions about the balance between speed and caution, resulting in a pipeline of novel therapies now moving through clinical trials, including third-generation 3CL protease inhibitors. A majority of those therapeutics are being developed in China.
References
Akha, Aging and the immune system: An overview, J Immunol Methods
Bali, Dhatt, Lal, Van Daalen, Sridhar, Off the back burner: diverse and gender-inclusive decision-making for COVID-19 response and recovery, BMJ Glob Health
Bazdyrev, Rusina, Panova, Novikov, Grishagin et al., Lung Fibrosis after COVID-19: Treatment Prospects, Pharmaceuticals
Bienvenu, Noonan, Wang, Peter, Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities, Cardiovasc Res
Bischof, Oertelt-Prigione, Morgan, Klein, Towards Precision Medicine: Inclusion of Sex and Gender Aspects in COVID-19 Clinical Studies-Acting Now before It Is Too Late-A Joint Call for Action, Int J Environ Res Public Health
Bischof, Wolfe, Klein, Clinical trials for COVID-19 should include sex as a variable, J Clin Invest
Brinkworth, Rusen, SARS-CoV-2 Is Not Special, but the Pandemic Is: The Ecology, Evolution, Policy, and Future of the Deadliest Pandemic in Living Memory, Annu Rev Anthropol
Cao, Gao, Bao, Feng, Mei et al., VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19, N Engl J Med
Cao, Li, Wang, Ran, Davalos et al., Accelerated biological aging in COVID-19 patients, Nat Commun
Cassidy, Dever, Stanbery, Edelman, Dworkin et al., FDA efficiency for approval process of COVID-19 therapeutics, Infect Agent Cancer
Cheng, Fu, Xu, Yip, Technology Platforms Are Revolutionizing Health Care Service Delivery in China, NEJM Catal
Consortium, Pan, Peto, Henao-Restrepo, Preziosi et al., Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
Couzin-Frankel, Antiviral pills could change pandemic's course, Science
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol
Eleftheriou, Amanatidou, Petrou, Geronikaki, In Silico Evaluation of the Effectivity of Approved Protease Inhibitors against the Main Protease of the Novel SARS-CoV-2 Virus, Molecules
Ferrucci, Fabbri, Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty, Nat Rev Cardiol
Froggatt, Heaton, Heaton, Development of a Fluorescence-Based, High-Throughput SARS-CoV-2 3CL pro Reporter Assay, J Virol
Galkin, Parish, Bischof, Zhang, Mamoshina et al., Increased Pace of Aging in COVID-Related Mortality, Life
Goetz, Choe, Hansell, Chen, Mcdowell et al., Substrate Specificity Profiling and Identification of a New Class of Inhibitor for the Major Protease of the SARS Coronavirus, Biochemistry
Grasselli, Pesenti, Cecconi, Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy, JAMA
Gurung, Ali, Lee, Farah, Km, Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach, Life Sci
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med
He, Hu, Huang, Wang, Zhang et al., Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: Insights from structures of protease and inhibitors, Int J Antimicrob Agents
Huang, Gu, Zhang, Cao, A glimpse into long COVID and symptoms -Authors' reply, Lancet Respir Med
Huang, Li, Gu, Zhang, Ren et al., Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study, Lancet Respir Med
Husain, Makadia, Valicherla, Riyazuddin, Gayen, Approaches to minimize the effects of P-glycoprotein in drug transport: A review, Drug Dev Res
Jin, Du, Xu, Deng, Liu et al., Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors, Nature
Jones, Bray, Khoo, Davey, Meaden et al., P-glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance?, AIDS
Kostopanagiotou, Schuurmans, Inci, Hage, COVID-19-related end stage lung disease: two distinct phenotypes, Ann Med
Kozlov, COVID drug Paxlovid was hailed as a game-changer, What happened? Nature
Kumar, Trivedi, Disease-drug and drugdrug interaction in COVID-19: Risk and assessment, Biomedicine & Pharmacotherapy
Loos, Beijnen, Schinkel, The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism?, Int J Mol Sci
Macías, Pinilla, Lao-Dominguez, Corma, Contreras-Macias et al., High rate of major drug-drug interactions of lopinavirritonavir for COVID-19 treatment, Sci Rep
Mansell, Dykgraaf, Kidd, Smith, Long COVID and older people, Lancet Healthy Longev
Mantovani, Morrone, Patrono, Santoro, Schiaffino et al., Long Covid: where we stand and challenges ahead, Cell Death Differ
Martins, Fazal, Oganesian, Shah, Stow et al., A commentary on the use of pharmacoenhancers in the pharmaceutical industry and the implication for DMPK drug discovery strategies, Xenobiotica
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Prescribing Nirmatrelvir-Ritonavir: How to Recognize and Manage Drug-Drug Interactions, Ann Intern Med
Marzolini, Kuritzkes, Marra, Boyle, Gibbons et al., Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications, Clin Pharmacol Ther
Mcgroder, Zhang, Choudhury, Salvatore, Souza et al., Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length, Thorax
Michelen, Manoharan, Elkheir, Cheng, Dagens et al., Characterising long COVID: a living systematic review, BMJ Glob Health
Mizrahi, Sudry, Flaks-Manov, Yehezkelli, Kalkstein et al., Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study, BMJ
Morrisette-Thomas, Cohen, Fülöp, Riesco, Legault et al., Inflamm-aging does not simply reflect increases in pro-inflammatory markers, Mech Ageing Dev
Muramatsu, Takemoto, Kim, Wang, Nishii et al., SARS-CoV 3CL protease cleaves its C-terminal autoprocessing site by novel subsite cooperativity, Proc Natl Acad Sci U S A
Mussini, Cozzi-Lepri, Menozzi, Meschiari, Franceschini et al., Better prognosis in females with severe COVID-19 pneumonia: possible role of inflammation as potential mediator, Clin Microbiol Infect
Mótyán, Mahdi, Hoffka, Tőzsér, Potential Resistance of SARS-CoV-2 Main Protease (Mpro) against Protease Inhibitors: Lessons Learned from HIV-1 Protease, Int J Mol Sci
Narayanan, Narwal, Majowicz, Varricchio, Toner et al., Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay, Commun Biol
Parums, Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients, Med Sci Monit
Robinson, Liew, Tanner, Grainger, Dwek et al., COVID-19 therapeutics: Challenges and directions for the future, Proc Natl Acad Sci U S A
Ross, Bortolussi-Courval, Hanula, Lee, Wilson et al., Drug Interactions With Nirmatrelvir-Ritonavir in Older Adults Using Multiple Medications, JAMA Netw Open
Rubin, From Positive to Negative to Positive Again-The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid, JAMA
Sarkar, Harty, Moeller, Klein, Erdman et al., The gut microbiome as a biomarker of differential susceptibility to SARS-CoV-2, Trends Mol Med
Scully, Gupta, Klein, Sex-biased clinical presentation and outcomes from COVID-19, Clinical Microbiology and Infection
Shamsi, Anwar, Mohd, Hussain, Mdt, Identification of high-affinity inhibitors of SARS-CoV-2 main protease: Towards the development of effective COVID-19 therapy, Virus Res
Shapiro, Morgan, Leng, Klein, Roadmap for Sex-Responsive Influenza and COVID-19 Vaccine Research in Older Adults, Frontiers in Aging
Song, Peng, Tang, Dai, Protease Inhibitor Use in COVID-19, SN Compr Clin Med
Stader, Kinvig, Battegay, Khoo, Owen et al., Analysis of Clinical Drug-Drug Interaction Data To Predict Magnitudes of Uncharacterized Interactions between Antiretroviral Drugs and Comedications, Antimicrob Agents Chemother
Tan, Liu, Zeng, China needs a scientific long COVID recovery-support platform, The Lancet
Ten-Caten, Gonzalez-Dias, Castro, Ogava, Giddaluru et al., In-depth analysis of laboratory parameters reveals the interplay between sex, age, and systemic inflammation in individuals with COVID-19, Int J Infect Dis
Vandyck, Deval, Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection, Curr Opin Virol
Verma, Kapoor, Das, Thakur, Potential Inhibitors of SARS-CoV-2 Main Protease (Mpro) Identified from the Library of FDA-Approved Drugs Using Molecular Docking Studies, Biomedicines
Wang, Berger, Davis, Kaelber, Volkow et al., COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022, medRxiv
Wang, Chen, Zhao, Feng, Rapid COVID-19 rebound in a severe COVID-19 patient during 20-day course of Paxlovid, J Infect
Wister, Speechley, COVID-19: Pandemic Risk, Resilience and Possibilities for Aging Research, Can J Aging
Zhang, Lu, Shi, The influence of telemedicine on capacity development in public primary hospitals in China: A scoping review, Clinical eHealth
Zhavoronkov, Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections, Aging
Zhu, Zhang, Lin, Lyu, Lu et al., Progress on SARS-CoV-2 3CLpro Inhibitors: Inspiration from SARS-CoV 3CLpro Peptidomimetics and Small-Molecule Anti-Inflammatory Compounds, Drug Des Devel Ther
DOI record:
{
"DOI": "10.14336/ad.2023.0318",
"ISSN": [
"2152-5250"
],
"URL": "http://dx.doi.org/10.14336/AD.2023.0318",
"author": [
{
"affiliation": [],
"family": "Bischof",
"given": "Evelyne",
"sequence": "first"
}
],
"container-title": "Aging and disease",
"container-title-short": "Aging and disease",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
3
]
],
"date-time": "2023-08-03T01:51:01Z",
"timestamp": 1691027461000
},
"deposited": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T22:03:24Z",
"timestamp": 1705701804000
},
"indexed": {
"date-parts": [
[
2024,
1,
20
]
],
"date-time": "2024-01-20T00:18:24Z",
"timestamp": 1705709904653
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"member": "5462",
"original-title": [],
"page": "1967",
"prefix": "10.14336",
"published": {
"date-parts": [
[
2023
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Aging and Disease",
"reference": [
{
"DOI": "10.1186/s13027-020-00338-z",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-1",
"unstructured": "Cassidy C, Dever D, Stanbery L, Edelman G, Dworkin L, Nemunaitis J (2020). FDA efficiency for approval process of COVID-19 therapeutics. Infect Agent Cancer, 15:73."
},
{
"DOI": "10.1016/j.ijid.2021.03.016",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-2",
"unstructured": "ten-Caten F, Gonzalez-Dias P, Castro Í, Ogava RLT, Giddaluru J, Silva JCS, et al. (2021). In-depth analysis of laboratory parameters reveals the interplay between sex, age, and systemic inflammation in individuals with COVID-19. Int J Infect Dis, 105:579-587."
},
{
"DOI": "10.3390/ijerph17103715",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-3",
"unstructured": "Bischof E, Oertelt-Prigione S, Morgan R, Klein S (2020). Towards Precision Medicine: Inclusion of Sex and Gender Aspects in COVID-19 Clinical Studies—Acting Now before It Is Too Late—A Joint Call for Action. Int J Environ Res Public Health, 17:3715."
},
{
"DOI": "10.1136/bmjgh-2020-002595",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-4",
"unstructured": "Bali S, Dhatt R, Lal A, Jama A, Van Daalen K, Sridhar D (2020). Off the back burner: diverse and gender-inclusive decision-making for COVID-19 response and recovery. BMJ Glob Health, 5:e002595."
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-5",
"unstructured": "Consortium W.H.O.S.T, Pan H, Peto R, Henao-Restrepo A.M, Preziosi M.P, Sathiyamoorthy V, et al. (2021). Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. N Engl J Med, 384:497-511."
},
{
"DOI": "10.1126/science.acx9605",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-6",
"unstructured": "Couzin-Frankel J (2021). Antiviral pills could change pandemic’s course. Science (1979), 374:799-800."
},
{
"DOI": "10.1016/j.cmi.2020.12.010",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-7",
"unstructured": "Mussini C, Cozzi-Lepri A, Menozzi M, Meschiari M, Franceschini E, Rogati C, et al. (2021). Better prognosis in females with severe COVID-19 pneumonia: possible role of inflammation as potential mediator. Clin Microbiol Infect, 27:1137-1144."
},
{
"DOI": "10.1093/cvr/cvaa284",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-8",
"unstructured": "Bienvenu LA, Noonan J, Wang X, Peter K (2020). Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res, 116:2197-2206."
},
{
"DOI": "10.1016/j.cmi.2021.03.027",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-9",
"unstructured": "Scully EP, Gupta A, Klein SL (2021). Sex-biased clinical presentation and outcomes from COVID-19. Clinical Microbiology and Infection, 27:1072-1073."
},
{
"DOI": "10.1172/JCI139306",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-10",
"unstructured": "Bischof E, Wolfe J, Klein SL (2020). Clinical trials for COVID-19 should include sex as a variable. J Clin Invest, 130:3350-3352."
},
{
"DOI": "10.3389/fragi.2022.836642",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-11",
"unstructured": "Shapiro JR, Morgan R, Leng SX, Klein SL (2022). Roadmap for Sex-Responsive Influenza and COVID-19 Vaccine Research in Older Adults. Frontiers in Aging."
},
{
"DOI": "10.12659/MSM.935952",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-12",
"unstructured": "Parums D V. (2022). Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients. Med Sci Monit, 28:e935952"
},
{
"DOI": "10.1146/annurev-anthro-041420-100047",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-13",
"unstructured": "Brinkworth JF, Rusen RM (2022). SARS-CoV-2 Is Not Special, but the Pandemic Is: The Ecology, Evolution, Policy, and Future of the Deadliest Pandemic in Living Memory. Annu Rev Anthropol, 51:527-548."
},
{
"DOI": "10.1017/S0714980820000215",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-14",
"unstructured": "Wister A, Speechley M (2020). COVID-19: Pandemic Risk, Resilience and Possibilities for Aging Research. Can J Aging, 39:344-347."
},
{
"DOI": "10.18632/aging.102988",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-15",
"unstructured": "Zhavoronkov A (2020). Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections. Aging, 12:6492-6510."
},
{
"DOI": "10.3390/life11080730",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-16",
"unstructured": "Galkin F, Parish A, Bischof E, Zhang J, Mamoshina P, Zhavoronkov A (2021). Increased Pace of Aging in COVID-Related Mortality. Life, 11:730."
},
{
"DOI": "10.1016/j.molmed.2021.09.009",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-17",
"unstructured": "Sarkar A, Harty S, Moeller AH, Klein SL, Erdman SE, Friston KJ, et al. (2021). The gut microbiome as a biomarker of differential susceptibility to SARS-CoV-2. Trends Mol Med, 27:1115-1134."
},
{
"DOI": "10.1038/s41569-018-0064-2",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-18",
"unstructured": "Ferrucci L, Fabbri E (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol, 15:505-522."
},
{
"DOI": "10.1016/j.mad.2014.06.005",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-19",
"unstructured": "Morrisette-Thomas V, Cohen AA, Fülöp T, Riesco É, Legault V, Li Q, et al. (2014). Inflamm-aging does not simply reflect increases in pro-inflammatory markers. Mech Ageing Dev, 139:49-57."
},
{
"DOI": "10.1016/j.jim.2018.08.005",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-20",
"unstructured": "Sadighi Akha AA (2018). Aging and the immune system: An overview. J Immunol Methods, 463:21-26."
},
{
"DOI": "10.1038/s41467-022-29801-8",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-21",
"unstructured": "Cao X, Li W, Wang T, Ran D, Davalos V, Planas-Serra L, et al. (2022). Accelerated biological aging in COVID-19 patients. Nat Commun, 13:2135."
},
{
"DOI": "10.1016/j.ceh.2022.10.001",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-22",
"unstructured": "Zhang J, Lu Q, Shi L (2022). The influence of telemedicine on capacity development in public primary hospitals in China: A scoping review. Clinical eHealth, 5:91-99."
},
{
"key": "key-10.14336/AD.2023.0318-23",
"unstructured": "Cheng T, Fu H, Xu D, Yip W (2022). Technology Platforms Are Revolutionizing Health Care Service Delivery in China. NEJM Catal, 4:4."
},
{
"DOI": "10.1016/S2666-7568(22)00245-8",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-24",
"unstructured": "Mansell V, Hall Dykgraaf S, Kidd M, Goodyear-Smith F (2022). Long COVID and older people. Lancet Healthy Longev, 3:e849-e854."
},
{
"DOI": "10.1136/bmj-2022-072529",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-25",
"unstructured": "Mizrahi B, Sudry T, Flaks-Manov N, Yehezkelli Y, Kalkstein N, Akiva P, et al. (2023). Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ, e072529."
},
{
"DOI": "10.1080/07853890.2022.2039954",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-26",
"unstructured": "Kostopanagiotou K, Schuurmans MM, Inci I, Hage R (2022). COVID-19-related end stage lung disease: two distinct phenotypes. Ann Med, 54:588-590."
},
{
"DOI": "10.3390/ph14080807",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-27",
"unstructured": "Bazdyrev E, Rusina P, Panova M, Novikov F, Grishagin I, Nebolsin V (2021). Lung Fibrosis after COVID-19: Treatment Prospects. Pharmaceuticals, 14:807."
},
{
"DOI": "10.1136/thoraxjnl-2021-217031",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-28",
"unstructured": "McGroder CF, Zhang D, Choudhury MA, Salvatore MM, D’Souza BM, Hoffman EA, et al. (2021). Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax, 76:1242-1245."
},
{
"DOI": "10.1038/s41579-022-00846-2",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-29",
"unstructured": "Davis HE, McCorkell L, Vogel JM, Topol EJ (2023). Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol, 21(3):133-146."
},
{
"DOI": "10.1136/bmjgh-2021-005427",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-30",
"unstructured": "Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. (2021). Characterising long COVID: a living systematic review. BMJ Glob Health, 6:e005427."
},
{
"DOI": "10.1038/s41418-022-01052-6",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-31",
"unstructured": "Mantovani A, Morrone MC, Patrono C, Santoro MG, Schiaffino S, Remuzzi G, et al. (2022). Long Covid: where we stand and challenges ahead. Cell Death Differ, 29(10):1891-1900."
},
{
"DOI": "10.1016/S2213-2600(22)00212-0",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-32",
"unstructured": "Huang L, Gu X, Zhang H, Cao B (2022). A glimpse into long COVID and symptoms - Authors’ reply. Lancet Respir Med, 10:e82."
},
{
"DOI": "10.1016/S2213-2600(22)00126-6",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-33",
"unstructured": "Huang L, Li X, Gu X, Zhang H, Ren L, Guo L, et al. (2022). Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med, 10:863-876."
},
{
"DOI": "10.1016/S0140-6736(23)00138-1",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-34",
"unstructured": "Tan H, Liu J, Zeng F (2023). China needs a scientific long COVID recovery-support platform. The Lancet, 401:344-345."
},
{
"DOI": "10.1016/j.jinf.2022.08.012",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-35",
"unstructured": "Wang Y, Chen X, Xiao W, Zhao D, Feng L (2022). Rapid COVID-19 rebound in a severe COVID-19 patient during 20-day course of Paxlovid. J Infect, 85:e134-e136."
},
{
"DOI": "10.1001/jama.2022.9925",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-36",
"unstructured": "Rubin R (2022). From Positive to Negative to Positive Again—The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid. JAMA, 327:2380."
},
{
"DOI": "10.1101/2022.06.21.22276724",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-37",
"unstructured": "Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R (2022). COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. medRxiv, 22:2022.06.21.22276724."
},
{
"DOI": "10.1038/d41586-022-04576-6",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-38",
"unstructured": "Kozlov M (2023). COVID drug Paxlovid was hailed as a game-changer. What happened? Nature, 613:224-225."
},
{
"DOI": "10.1056/NEJMoa2208822",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-39",
"unstructured": "Cao Z, Gao W, Bao H, Feng H, Mei S, Chen P, et al. (2023). VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19. N Engl J Med, 388:406-417."
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-40",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. (2022). Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med, 386:1397-1408."
},
{
"DOI": "10.3390/biomedicines11010085",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-41",
"unstructured": "Verma DK, Kapoor S, Das S, Thakur KG (2022). Potential Inhibitors of SARS-CoV-2 Main Protease (Mpro) Identified from the Library of FDA-Approved Drugs Using Molecular Docking Studies. Biomedicines, 11:85."
},
{
"DOI": "10.1016/j.lfs.2020.117831",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-42",
"unstructured": "Gurung AB, Ali MA, Lee J, Farah MA, Al-Anazi KM (2020). Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach. Life Sci, 255:117831."
},
{
"DOI": "10.1128/JVI.01265-20",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-43",
"unstructured": "Froggatt HM, Heaton BE, Heaton NS (2020). Development of a Fluorescence-Based, High-Throughput SARS-CoV-2 3CL pro Reporter Assay. J Virol, 94(22):e01265-20."
},
{
"DOI": "10.1016/j.virusres.2020.198102",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-44",
"unstructured": "Mohammad T, Shamsi A, Anwar S, Umair Mohd, Hussain A, Rehman MdT, et al. (2020). Identification of high-affinity inhibitors of SARS-CoV-2 main protease: Towards the development of effective COVID-19 therapy. Virus Res, 288:198102."
},
{
"DOI": "10.1007/s42399-020-00448-0",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-45",
"unstructured": "Song Y, Peng W, Tang D, Dai Y (2020). Protease Inhibitor Use in COVID-19. SN Compr Clin Med, 2:1436-1443."
},
{
"DOI": "10.1001/jama.2020.4031",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-46",
"unstructured": "Grasselli G, Pesenti A, Cecconi M (2020). Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy. JAMA, 323:1545."
},
{
"DOI": "10.1038/s42003-022-03090-9",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-47",
"unstructured": "Narayanan A, Narwal M, Majowicz SA, Varricchio C, Toner SA, Ballatore C, et al. (2022). Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay. Commun Biol, 5:169."
},
{
"DOI": "10.1038/s41586-020-2223-y",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-48",
"unstructured": "Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. (2020). Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature, 582:289-293."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106055",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-49",
"unstructured": "He J, Hu L, Huang X, Wang C, Zhang Z, Wang Y, et al. (2020). Potential of coronavirus 3C-like protease inhibitors for the development of new anti-SARS-CoV-2 drugs: Insights from structures of protease and inhibitors. Int J Antimicrob Agents, 56:106055."
},
{
"DOI": "10.2147/DDDT.S359009",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-50",
"unstructured": "Zhu J, Zhang H, Lin Q, Lyu J, Lu L, Chen H, et al. (2022). Progress on SARS-CoV-2 3CLpro Inhibitors: Inspiration from SARS-CoV 3CLpro Peptidomimetics and Small-Molecule Anti-Inflammatory Compounds. Drug Des Devel Ther, 16:1067-1082."
},
{
"DOI": "10.3390/molecules25112529",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-51",
"unstructured": "Eleftheriou P, Amanatidou D, Petrou A, Geronikaki A (2020). In Silico Evaluation of the Effectivity of Approved Protease Inhibitors against the Main Protease of the Novel SARS-CoV-2 Virus. Molecules, 25:2529."
},
{
"DOI": "10.1073/pnas.1601327113",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-52",
"unstructured": "Muramatsu T, Takemoto C, Kim Y-T, Wang H, Nishii W, Terada T, et al. (2016). SARS-CoV 3CL protease cleaves its C-terminal autoprocessing site by novel subsite cooperativity. Proc Natl Acad Sci U S A, 113:12997-13002."
},
{
"DOI": "10.1021/bi0621415",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-53",
"unstructured": "Goetz DH, Choe Y, Hansell E, Chen YT, McDowell M, Jonsson CB, et al. (2007). Substrate Specificity Profiling and Identification of a New Class of Inhibitor for the Major Protease of the SARS Coronavirus. Biochemistry, 46:8744-8752."
},
{
"DOI": "10.1080/00498254.2022.2130838",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-54",
"unstructured": "Martins V, Fazal L, Oganesian A, Shah A, Stow J, Walton H, et al. (2022). A commentary on the use of pharmacoenhancers in the pharmaceutical industry and the implication for DMPK drug discovery strategies. Xenobiotica, 52:786-796."
},
{
"DOI": "10.3390/ijms23179866",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-55",
"unstructured": "Loos NHC, Beijnen JH, Schinkel AH (2022). The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism? Int J Mol Sci, 23:9866."
},
{
"DOI": "10.1016/j.biopha.2021.111642",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-56",
"unstructured": "Kumar D, Trivedi N (2021). Disease-drug and drug-drug interaction in COVID-19: Risk and assessment. Biomedicine & Pharmacotherapy, 139:111642."
},
{
"DOI": "10.1002/cpt.2646",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-57",
"unstructured": "Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, Flexner C, et al. (2022). Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications. Clin Pharmacol Ther, 112:1191-1200."
},
{
"DOI": "10.1128/AAC.00717-18",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-58",
"unstructured": "Stader F, Kinvig H, Battegay M, Khoo S, Owen A, Siccardi M, et al. (2018). Analysis of Clinical Drug-Drug Interaction Data To Predict Magnitudes of Uncharacterized Interactions between Antiretroviral Drugs and Comedications. Antimicrob Agents Chemother, 62(7):e00717-18."
},
{
"DOI": "10.7326/M22-0281",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-59",
"unstructured": "Marzolini C, Kuritzkes DR, Marra F, Boyle A, Gibbons S, Flexner C, et al. (2022). Prescribing Nirmatrelvir-Ritonavir: How to Recognize and Manage Drug-Drug Interactions. Ann Intern Med, 175:744-746."
},
{
"DOI": "10.1001/jamanetworkopen.2022.20184",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-60",
"unstructured": "Ross SB, Bortolussi-Courval É, Hanula R, Lee TC, Goodwin Wilson M, McDonald EG (2022). Drug Interactions With Nirmatrelvir-Ritonavir in Older Adults Using Multiple Medications. JAMA Netw Open, 5:e2220184."
},
{
"DOI": "10.1038/s41598-020-78029-3",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-61",
"unstructured": "Macías J, Pinilla A, Lao-Dominguez FA, Corma A, Contreras-Macias E, González-Serna A, et al. (2020). High rate of major drug-drug interactions of lopinavir-ritonavir for COVID-19 treatment. Sci Rep, 10:20958."
},
{
"DOI": "10.3390/ijms23073507",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-62",
"unstructured": "Mótyán JA, Mahdi M, Hoffka G, Tőzsér J (2022). Potential Resistance of SARS-CoV-2 Main Protease (Mpro) against Protease Inhibitors: Lessons Learned from HIV-1 Protease. Int J Mol Sci, 23:3507."
},
{
"DOI": "10.1097/00002030-200107270-00004",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-63",
"unstructured": "Jones K, Bray PG, Khoo SH, Davey RA, Meaden ER, Ward SA, et al. (2001). P-glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS, 15:1353-1358."
},
{
"DOI": "10.1002/ddr.21918",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-64",
"unstructured": "Husain A, Makadia V, Valicherla GR, Riyazuddin M, Gayen JR (2022). Approaches to minimize the effects of P-glycoprotein in drug transport: A review. Drug Dev Res, 83:825-841."
},
{
"DOI": "10.1016/j.coviro.2021.04.006",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-65",
"unstructured": "Vandyck K, Deval J (2021). Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr Opin Virol, 49:36-40."
},
{
"DOI": "10.1073/pnas.2119893119",
"doi-asserted-by": "crossref",
"key": "key-10.14336/AD.2023.0318-66",
"unstructured": "Robinson PC, Liew DFL, Tanner HL, Grainger JR, Dwek RA, Reisler RB, et al. (2022). COVID-19 therapeutics: Challenges and directions for the future. Proc Natl Acad Sci U S A, 119(15):e2119893119."
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.aginganddisease.org/EN/10.14336/AD.2023.0318"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Neurology (clinical)",
"Geriatrics and Gerontology",
"Pathology and Forensic Medicine"
],
"subtitle": [],
"title": "Mitigating COVID-19 Mortality and Morbidity in China's Aging Population: A Focus on Available Medications and Future Developments",
"type": "journal-article",
"volume": "14"
}