2024 Clinical Practice Guideline Update by the Infectious Diseases Society of America on the Management of COVID-19: Anti-SARS-CoV-2 Neutralizing Antibody Pemivibart for Pre-exposure Prophylaxis
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae435, Oct 2024
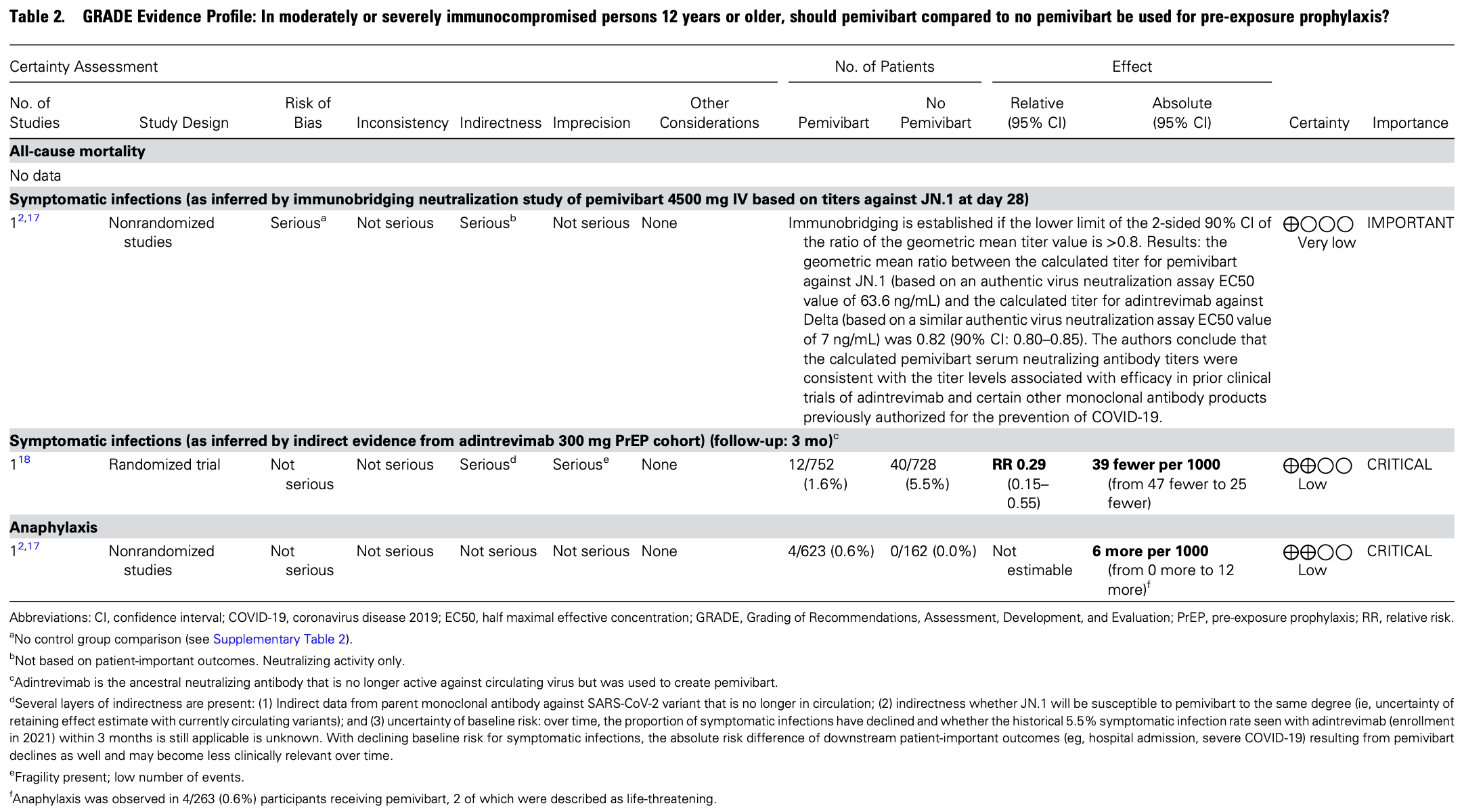
Update to IDSA clinical practice guidelines on the treatment and management of COVID-19, providing a conditional recommendation for pre-exposure prophylaxis with pemivibart, an anti-SARS-CoV-2 neutralizing antibody, in moderately or severely immunocompromised individuals aged 12 years or older at risk for progression to severe COVID-19, when predominant regional variants are susceptible to the agent. The recommendation is based on low certainty of evidence derived from immunobridging studies. Serious adverse events included a 0.6% risk of anaphylaxis with pemivibart.
Efficacy is variant dependent. In Vitro research shows reduced efficacy against KP.3.1.1, KP.1.1, LB.1, KP.3.3, and XEC variants1-4.
1.
Xie et al., Molecular Basis of High-Blood-Pressure-Enhanced and High-Fever-Temperature-Weakened Receptor-Binding Domain/Peptidase Domain Binding: A Molecular Dynamics Simulation Study, International Journal of Molecular Sciences, doi:10.3390/ijms26073250.
2.
Wang et al., Activity of Research-Grade Pemivibart against Recent SARS-CoV-2 JN.1 Sublineages, New England Journal of Medicine, doi:10.1056/NEJMc2410203.
Bhimraj et al., 29 Oct 2024, USA, peer-reviewed, 26 authors.
Contact: practiceguidelines@idsociety.org.
2024 Clinical Practice Guideline Update by the Infectious Diseases Society of America on the Management of COVID-19: Anti-SARS-CoV-2 Neutralizing Antibody Pemivibart for Pre-exposure Prophylaxis
Clinical Infectious Diseases, doi:10.1093/cid/ciae435
This article provides a focused update to the clinical practice guideline on the treatment and management of patients with coronavirus disease 2019, developed by the Infectious Diseases Society of America. The guideline panel presents a recommendation on the use of the anti-severe acute respiratory syndrome coronavirus 2 neutralizing antibody pemivibart as pre-exposure prophylaxis. The recommendation is based on evidence derived from a systematic review and adheres to a standardized methodology for rating the certainty of evidence and strength of recommendation according to the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach. Information on pemivibart is included in the U.S. Food and Drug Administration
and Gilead Sciences; K.W.C. for Pardes Biosciences (concluded); E.D. for Gilead Sciences; D.V.G. for Gilead Sciences and Merck; A.K. for Shionogi; S.S. for Pfizer; P.T. for Merck and Shionogi. Importantly, these companies have developed COVID-19 treatment (but not prophylaxis) agents. No disclosures reported for all other authors including the chair and vice chair. Additional information. More detailed information on the analysis and development of recommendations is available in the Supplementary Material.
References
Bhimraj, Morgan, Shumaker, Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 (September 2022), Clin Infect Dis
Evans, Dube, Lu, Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational populationbased INFORM study, Lancet Reg Health Eur
Follmann, Brien, Fintzi, Examining protective effects of SARS-CoV-2 neutralizing antibodies after vaccination or monoclonal antibody administration, Nat Commun
Guyatt, Oxman, Vist, GRADE: an emerging consensus on rating quality of evidence and strength of recommendations, BMJ
Invivyd, A study to evaluate the efficacy and safety of VYD222 for prevention of COVID-19 (CANOPY)
Ison, Weinstein, Dobryanska, Prevention of COVID-19 following a single intramuscular administration of adintrevimab: results from a phase 2/3 randomized, double-blind, placebo-controlled trial (EVADE), Open Forum Infect Dis
Levin, Ustianowski, Wit, Intramuscular AZD7442 (tixagevimabcilgavimab) for prevention of COVID-19, N Eng J Med
Loo, Tamney, Arends, The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans, Sci Transl Med
Rappazzo, Tse, Kaku, Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody, Science
Solera, Árbol, Mittal, Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023, Am J Transplant
Stadler, Burgess, Schlub, Monoclonal antibody levels and protection from COVID-19, Nat Commun
DOI record:
{
"DOI": "10.1093/cid/ciae435",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciae435",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>This article provides a focused update to the clinical practice guideline on the treatment and management of patients with coronavirus disease 2019, developed by the Infectious Diseases Society of America. The guideline panel presents a recommendation on the use of the anti–severe acute respiratory syndrome coronavirus 2 neutralizing antibody pemivibart as pre-exposure prophylaxis. The recommendation is based on evidence derived from a systematic review and adheres to a standardized methodology for rating the certainty of evidence and strength of recommendation according to the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach. Information on pemivibart is included in the U.S. Food and Drug Administration Emergency Use Authorization for this agent.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Houston Methodist Hospital , Houston, Texas ,",
"place": [
"USA"
]
}
],
"family": "Bhimraj",
"given": "Adarsh",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine, Case Western Reserve University, School of Medicine , Cleveland, Ohio ,",
"place": [
"USA"
]
},
{
"name": "Department of Medicine, VA Northeast Ohio Healthcare System , Cleveland, Ohio ,",
"place": [
"USA"
]
}
],
"family": "Falck-Ytter",
"given": "Yngve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School , Boston, Massachusetts ,",
"place": [
"USA"
]
},
{
"name": "Infectious Diseases Division, Department of Medicine, Massachusetts General Hospital , Boston, Massachusetts ,",
"place": [
"USA"
]
}
],
"family": "Kim",
"given": "Arthur Y",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School , Boston, Massachusetts ,",
"place": [
"USA"
]
},
{
"name": "Department of Medicine, Brigham and Women's Hospital , Boston, Massachusetts ,",
"place": [
"USA"
]
}
],
"family": "Li",
"given": "Jonathan Z",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School , Boston, Massachusetts ,",
"place": [
"USA"
]
},
{
"name": "Department of Medicine, Brigham and Women's Hospital , Boston, Massachusetts ,",
"place": [
"USA"
]
}
],
"family": "Baden",
"given": "Lindsey R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine , Aurora, Colorado ,",
"place": [
"USA"
]
}
],
"family": "Johnson",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University , Palo Alto, California ,",
"place": [
"USA"
]
}
],
"family": "Shafer",
"given": "Robert W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Johns Hopkins University School of Medicine , Baltimore, Maryland ,",
"place": [
"USA"
]
}
],
"family": "Shoham",
"given": "Shmuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Pennsylvania , Philadelphia, Pennsylvania ,",
"place": [
"USA"
]
}
],
"family": "Tebas",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, UT Southwestern/VA North Texas Health Care System , Dallas, Texas ,",
"place": [
"USA"
]
}
],
"family": "Bedimo",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Queen Mary Hospital, Li Ka Shing Faculty of Medicine, The University of Hong Kong , Pokfulam, Hong Kong Special Administrative Region ,",
"place": [
"China"
]
}
],
"family": "Cheng",
"given": "Vincent Chi-Chung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, David Geffen School of Medicine, University of California Los Angeles , Los Angeles, California ,",
"place": [
"USA"
]
}
],
"family": "Chew",
"given": "Kara W",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology and Critical Care, Children's Hospital of Philadelphia/University of Pennsylvania , Philadelphia, Pennsylvania ,",
"place": [
"USA"
]
}
],
"family": "Chiotos",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Harbor-UCLA Medical Center , Torrance, California ,",
"place": [
"USA"
]
}
],
"family": "Daar",
"given": "Eric S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, New York-Presbyterian Hospital , New York, New York ,",
"place": [
"USA"
]
}
],
"family": "Dzierba",
"given": "Amy L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Epidemiology and Biostatistics, UCSF , San Francisco, California ,",
"place": [
"USA"
]
}
],
"family": "Glidden",
"given": "David V",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Departments of Medicine and Obstetrics and Gynecology, Brown University , Providence, Rhode Island ,",
"place": [
"USA"
]
}
],
"family": "Hardy",
"given": "Erica J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Emory University , Atlanta, Georgia ,",
"place": [
"USA"
]
}
],
"family": "Martin",
"given": "Greg S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy, Children's Hospital Colorado , Aurora, Colorado ,",
"place": [
"USA"
]
}
],
"family": "MacBrayne",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Northwestern University Feinberg School of Medicine , Chicago, Illinois ,",
"place": [
"USA"
]
}
],
"family": "Nadig",
"given": "Nandita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Antimicrobial Stewardship Program and Division of Infectious Diseases, Boston Children's Hospital , Boston, Massachusetts ,",
"place": [
"USA"
]
},
{
"name": "Department of Pediatrics, Harvard Medical School , Boston, Massachusetts ,",
"place": [
"USA"
]
}
],
"family": "Nakamura",
"given": "Mari M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Case Western Reserve University, School of Medicine , Cleveland, Ohio ,",
"place": [
"USA"
]
},
{
"name": "Department of Medicine, VA Northeast Ohio Healthcare System , Cleveland, Ohio ,",
"place": [
"USA"
]
}
],
"family": "Shumaker",
"given": "Amy Hirsch",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, UCSF/VA , San Francisco, California ,",
"place": [
"USA"
]
}
],
"family": "Tien",
"given": "Phyllis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Affairs and Practice Guidelines, Infectious Diseases Society of America , Arlington, Virginia ,",
"place": [
"USA"
]
}
],
"family": "Loveless",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Case Western Reserve University, School of Medicine , Cleveland, Ohio ,",
"place": [
"USA"
]
},
{
"name": "Department of Health Research Methods, Evidence, and Impact, McMaster University , Hamilton, Ontario ,",
"place": [
"Canada"
]
}
],
"family": "Morgan",
"given": "Rebecca L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Harvard Medical School , Boston, Massachusetts ,",
"place": [
"USA"
]
},
{
"name": "Infectious Diseases Division, Department of Medicine, Massachusetts General Hospital , Boston, Massachusetts ,",
"place": [
"USA"
]
}
],
"family": "Gandhi",
"given": "Rajesh T",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
8,
23
]
],
"date-time": "2024-08-23T03:19:10Z",
"timestamp": 1724383150000
},
"deposited": {
"date-parts": [
[
2024,
10,
29
]
],
"date-time": "2024-10-29T21:36:11Z",
"timestamp": 1730237771000
},
"funder": [
{
"DOI": "10.13039/100006292",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100006292",
"id-type": "DOI"
}
],
"name": "Infectious Diseases Society of America"
},
{
"DOI": "10.13039/100000030",
"award": [
"6 NU50CK000477-04-01"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000030",
"id-type": "DOI"
}
],
"name": "Centers for Disease Control and Prevention"
}
],
"indexed": {
"date-parts": [
[
2024,
10,
29
]
],
"date-time": "2024-10-29T22:10:10Z",
"timestamp": 1730239810528,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
10,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
29
]
],
"date-time": "2024-10-29T00:00:00Z",
"timestamp": 1730160000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae435/60212376/ciae435.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae435/60212376/ciae435.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
10,
29
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
29
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"author": "Centers for Disease Control and Prevention. COVID Data Tracker, Variants and Genomic Surveillance",
"key": "2024102921355711100_ciae435-B1"
},
{
"author": "U. S. Food and Drug Administration",
"key": "2024102921355711100_ciae435-B2"
},
{
"DOI": "10.1016/j.lanepe.2023.100747",
"article-title": "Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study",
"author": "Evans",
"doi-asserted-by": "crossref",
"first-page": "100747",
"journal-title": "Lancet Reg Health Eur",
"key": "2024102921355711100_ciae435-B3",
"volume": "35",
"year": "2023"
},
{
"DOI": "10.1016/j.ajt.2024.03.011",
"article-title": "Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023",
"author": "Solera",
"doi-asserted-by": "crossref",
"first-page": "1303",
"journal-title": "Am J Transplant",
"key": "2024102921355711100_ciae435-B4",
"volume": "24",
"year": "2024"
},
{
"DOI": "10.1126/scitranslmed.abl8124",
"article-title": "The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans",
"author": "Loo",
"doi-asserted-by": "crossref",
"first-page": "eabl8124",
"journal-title": "Sci Transl Med",
"key": "2024102921355711100_ciae435-B5",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1126/science.abf4830",
"article-title": "Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody",
"author": "Rappazzo",
"doi-asserted-by": "crossref",
"first-page": "823",
"journal-title": "Science",
"key": "2024102921355711100_ciae435-B6",
"volume": "371",
"year": "2021"
},
{
"author": "U. S. Food and Drug Administration",
"key": "2024102921355711100_ciae435-B7"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19",
"author": "Levin",
"doi-asserted-by": "crossref",
"first-page": "2188",
"journal-title": "N Eng J Med",
"key": "2024102921355711100_ciae435-B8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41467-023-39292-w",
"article-title": "Examining protective effects of SARS-CoV-2 neutralizing antibodies after vaccination or monoclonal antibody administration",
"author": "Follmann",
"doi-asserted-by": "crossref",
"first-page": "3605",
"journal-title": "Nat Commun",
"key": "2024102921355711100_ciae435-B9",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41467-023-40204-1",
"article-title": "Monoclonal antibody levels and protection from COVID-19",
"author": "Stadler",
"doi-asserted-by": "crossref",
"first-page": "4545",
"journal-title": "Nat Commun",
"key": "2024102921355711100_ciae435-B10",
"volume": "14",
"year": "2023"
},
{
"author": "U. S. Food and Drug Administration",
"key": "2024102921355711100_ciae435-B11"
},
{
"author": "U. S. Food and Drug Administration",
"key": "2024102921355711100_ciae435-B12"
},
{
"article-title": "WHO consultation on immunobridging.",
"author": "World Health Organization",
"key": "2024102921355711100_ciae435-B13"
},
{
"DOI": "10.1093/cid/ciac724",
"article-title": "Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19 (September 2022)",
"author": "Bhimraj",
"doi-asserted-by": "crossref",
"first-page": "e250",
"journal-title": "Clin Infect Dis",
"key": "2024102921355711100_ciae435-B14",
"volume": "78",
"year": "2024"
},
{
"DOI": "10.1136/bmj.39489.470347.AD",
"article-title": "GRADE: an emerging consensus on rating quality of evidence and strength of recommendations",
"author": "Guyatt",
"doi-asserted-by": "crossref",
"first-page": "924",
"journal-title": "BMJ",
"key": "2024102921355711100_ciae435-B15",
"volume": "336",
"year": "2008"
},
{
"author": "Infectious Diseases Society of America",
"key": "2024102921355711100_ciae435-B16"
},
{
"key": "2024102921355711100_ciae435-B17"
},
{
"DOI": "10.1093/ofid/ofad314",
"article-title": "Prevention of COVID-19 following a single intramuscular administration of adintrevimab: results from a phase 2/3 randomized, double-blind, placebo-controlled trial (EVADE)",
"author": "Ison",
"doi-asserted-by": "crossref",
"first-page": "ofad314",
"journal-title": "Open Forum Infect Dis",
"key": "2024102921355711100_ciae435-B18",
"volume": "10",
"year": "2023"
},
{
"key": "2024102921355711100_ciae435-B19"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae435/7849632"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "2024 Clinical Practice Guideline Update by the Infectious Diseases Society of America on the Management of COVID-19: Anti-SARS-CoV-2 Neutralizing Antibody Pemivibart for Pre-exposure Prophylaxis",
"type": "journal-article"
}