Management of immunosuppression in lung transplant recipients and COVID-19 outcomes: an observational retrospective cohort-study
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-09269-1, May 2024
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 91 lung transplant recipients with COVID-19 showing no significant difference in poor outcomes with casirivimab/imdevimab or tixagevimab/cilgavimab prophylaxis in univariate analysis.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Study covers casirivimab/imdevimab and tixagevimab/cilgavimab.
|
oxygen increase, ICU, or mortality, 67.4% higher, RR 1.67, p = 0.31, treatment 5 of 17 (29.4%), control 13 of 74 (17.6%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Bes-Berlandier et al., 28 May 2024, retrospective, France, peer-reviewed, median age 51.0, 10 authors, study period March 2020 - April 2022.
Contact: cassirnadim@gmail.com.
Management of immunosuppression in lung transplant recipients and COVID-19 outcomes: an observational retrospective cohort-study
BMC Infectious Diseases, doi:10.1186/s12879-024-09269-1
Background The aim of this study was to assess the impact of immunosuppression management on coronavirus disease 2019 (COVID-19) outcomes.
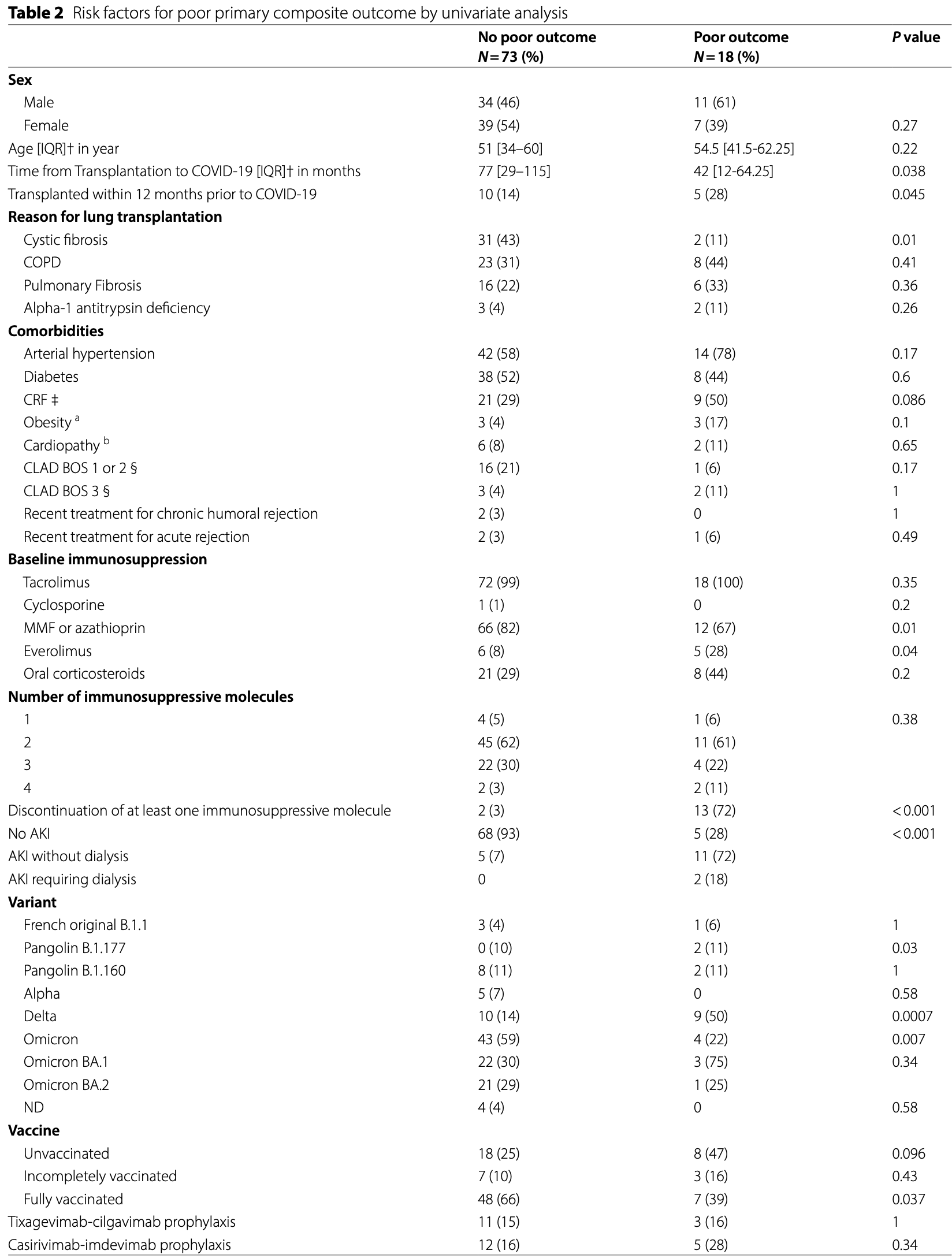
Methods We performed a single-center retrospective study in a cohort of 358 lung transplant recipients (LTx) over the period from March 2020 to April 2022. All included symptomatic patients had at least one positive SARS-CoV-2 rt-PCR. We used a composite primary outcome for COVID-19 including increased need for oxygen since the hospital admission, ICU transfer, and in-hospital mortality. We assessed by univariate and multivariate analyses the risk factors for poor outcomes. Results Overall, we included 91 LTx who contracted COVID-19. The COVID-19 in-hospital mortality rate reached 4.4%. By hierarchical clustering, we found a strong and independent association between the composite poor outcome and the discontinuation of at least one immunosuppressive molecule among tacrolimus, cyclosporine, mycophenolate mofetil, and everolimus. Obesity (OR = 16, 95%CI (1.96; 167), p = 0.01) and chronic renal failure (OR = 4.6, 95%CI (1.4; 18), p = 0.01) were also independently associated with the composite poor outcome. Conversely, full vaccination was protective (OR = 0.23, 95%CI (0.046; 0.89), p = 0.047).
Conclusion The administration of immunosuppressive drugs such as tacrolimus, cyclocporine or everolimus can have a protective effect in LTx with COVID-19, probably related to their intrinsic antiviral capacity.
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12879-024-09269-1.
Supplementary Material 1
Author contributions
Data availability The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations Ethics approval and consent to participate Due to the retrospective nature of the study, the need for informed consent was waived by the Ethics Committee of the Société de Pneumologie de Langue Française (CEPRO) (number CEPRO2023-036)
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aversa, Benvenuto, Anderson, COVID-19 in lung transplant recipients: a single center case series from New York City, Am J Transpl
Avery, Chiang, Marr, Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: a retrospective cohort, Am J Transpl
Belli, Fondevila, Cortesi, Protective role of Tacrolimus, Deleterious Role of Age and comorbidities in Liver Transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study, Gastroenterology
Bösch, Börner, Kemmner, Attenuated early inflammatory response in solid organ recipients with COVID-19, Clin Transpl
Carbajo-Lozoya, Ma-Lauer, Malešević, Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir, Virus Res
Choi, Li, Hao, Yao, Guan, Immunosuppressive therapy and COVID-19 infection in patients with NMOSD, Immun Inflamm Dis
Coiffard, Lepper, Prud'homme, Management of lung transplantation in the COVID-19 era-An international survey, Am J Transpl
Colson, Fournier, Chaudet, Analysis of SARS-CoV-2 variants from 24,181 patients exemplifies the role of globalization and zoonosis in Pandemics, Front Microbiol
De Andrade, De Sandes-Freitas, Requião-Moura, Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients, Am J Transpl
Desmazes-Dufeu, Coltey, Amari, Discordant courses of COVID-19 in a cohabiting couple of lung transplant recipients, Transpl Infect Dis
Forchette, Sebastian, Liu, A Comprehensive Review of COVID-19 Virology, vaccines, variants, and therapeutics, Curr Med Sci
Garcia, Sharma, Ramaiah, Antiviral drug screen identifies DNAdamage response inhibitor as potent blocker of SARS-CoV-2 replication, Cell Rep
Gatti, Rinaldi, Bussini, Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis, Clin Microbiol Infect
Ge, Li, Wu, Candido, Wei, Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: a population-based cohort study, PLoS ONE
Heldman, Kates, Safa, COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study, Am J Transpl
Husson, Josse, Lê, FactoMineR: an R Package for Multivariate Analysis, J Stat Softw
Kamp, Hinrichs, Fuge, Ewen, Gottlieb, COVID-19 in lung transplant recipients-risk prediction and outcomes, PLoS ONE
Karruli, Spiezia, Boccia, Effect of immunosuppression maintenance in solid organ transplant recipients with COVID-19: systematic review and meta-analysis, Transpl Infect Dis
Liddicoat, Lavelle, Modulation of innate immunity by cyclosporine A, Biochem Pharmacol
Manuel, Estabrook, American Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice, Clin Transpl
Merli, Pasulo, Perricone, Impact of immunosuppressive therapy on the severity of COVID-19 in solid organ transplant recipients, J Infect
Messika, Eloy, Roux, COVID-19 in lung transplant recipients, Transplantation
Nguyen, Houhamdi, Meddeb, Colson, Gautret, Reinfection with SARS-CoV-2 Omicron BA.4 and BA.5 variants, J Med Virol
Ogando, Metscher, Moes, The cyclophilin-dependent calcineurin inhibitor voclosporin inhibits SARS-CoV-2 replication in Cell Culture, Transpl Int
Pinchera, Spirito, Buonomo, mTOR Inhibitor Use Is Associated With a Favorable Outcome of COVID-19 in Patients of Kidney Transplant: Results of a Retrospective Study, Frontiers in Medicine, doi:10.3389/fmed.2022.852973
Pizzorno, Padey, Dubois, In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2, Antiviral Res
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine, N Engl J Med
Ringer, Azmy, Kaman, A retrospective matched cohort singlecenter study evaluating outcomes of COVID-19 and the impact of immunomodulation on COVID-19-related cytokine release syndrome in solid organ transplant recipients, Transpl Infect Dis
Roosma, Van Gemert, De Zwart, The effect of COVID-19 on transplant function and development of CLAD in lung transplant patients: a multicenter experience, J Heart Lung Transpl
Sabbatini, Ruggiero, Palatucci, Oscillatory mTOR inhibition and Treg increase in kidney transplantation, Clin Exp Immunol
Terrazzano, Rubino, Palatucci, Giovazzino, Carriero et al., An Open question: is it rational to inhibit the mtor-dependent pathway as COVID-19 therapy?, Front Pharmacol
Thakur, Bhola, Thakur, Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe, Infection
Trapani, Masiero, Puoti, Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide populationbased study, Am J Transpl
Trindade, Chapin, Gannon, Clinical course of SARS-CoV-2 infection and recovery in lung transplant recipients, Transpl Infect Dis
Verleden, Glanville, Lease, Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT, J Heart Lung Transpl
Weder, Kadl, Longitudinal impact of COVID-19 on lung function in lung transplant recipients: time to stop worrying?, Transpl Infect Dis
Willicombe, Thomas, Mcadoo, COVID-19 and calcineurin inhibitors: should they get left out in the storm?, J Am Soc Nephrol
Zimmermann, Glueck, Fertmann, COVID-19 in recent lung transplant recipients: clinical outcomes and management strategies, Transpl Proc
DOI record:
{
"DOI": "10.1186/s12879-024-09269-1",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-09269-1",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The aim of this study was to assess the impact of immunosuppression management on coronavirus disease 2019 (COVID-19) outcomes.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We performed a single-center retrospective study in a cohort of 358 lung transplant recipients (LTx) over the period from March 2020 to April 2022. All included symptomatic patients had at least one positive SARS-CoV-2 rt-PCR. We used a composite primary outcome for COVID-19 including increased need for oxygen since the hospital admission, ICU transfer, and in-hospital mortality. We assessed by univariate and multivariate analyses the risk factors for poor outcomes.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Overall, we included 91 LTx who contracted COVID-19. The COVID-19 in-hospital mortality rate reached 4.4%. By hierarchical clustering, we found a strong and independent association between the composite poor outcome and the discontinuation of at least one immunosuppressive molecule among tacrolimus, cyclosporine, mycophenolate mofetil, and everolimus. Obesity (OR = 16, 95%CI (1.96; 167), <jats:italic>p</jats:italic> = 0.01) and chronic renal failure (OR = 4.6, 95%CI (1.4; 18), <jats:italic>p</jats:italic> = 0.01) were also independently associated with the composite poor outcome. Conversely, full vaccination was protective (OR = 0.23, 95%CI (0.046; 0.89), <jats:italic>p</jats:italic> = 0.047).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The administration of immunosuppressive drugs such as tacrolimus, cyclocporine or everolimus can have a protective effect in LTx with COVID-19, probably related to their intrinsic antiviral capacity.</jats:p>\n </jats:sec>",
"alternative-id": [
"9269"
],
"article-number": "536",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "28 May 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Due to the retrospective nature of the study, the need for informed consent was waived by the Ethics Committee of the Société de Pneumologie de Langue Française (CEPRO) (number CEPRO2023-036)"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Bes-Berlandier",
"given": "Hugo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Coiffard",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bermudez",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Demazes-dufeu",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coltey",
"given": "Bérengère",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boschi",
"given": "Céline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colson",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hraiech",
"given": "Sami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reynaud-Gaubert",
"given": "Martine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cassir",
"given": "Nadim",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
5,
28
]
],
"date-time": "2024-05-28T15:08:55Z",
"timestamp": 1716908935000
},
"deposited": {
"date-parts": [
[
2024,
5,
28
]
],
"date-time": "2024-05-28T15:09:06Z",
"timestamp": 1716908946000
},
"indexed": {
"date-parts": [
[
2024,
5,
28
]
],
"date-time": "2024-05-28T17:40:18Z",
"timestamp": 1716918018350
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
5,
28
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
28
]
],
"date-time": "2024-05-28T00:00:00Z",
"timestamp": 1716854400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
28
]
],
"date-time": "2024-05-28T00:00:00Z",
"timestamp": 1716854400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09269-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-09269-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09269-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
5,
28
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
28
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1371/journal.pone.0258154",
"author": "E Ge",
"doi-asserted-by": "publisher",
"first-page": "e0258154",
"journal-title": "PLoS ONE",
"key": "9269_CR1",
"unstructured": "Ge E, Li Y, Wu S, Candido E, Wei X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: a population-based cohort study. PLoS ONE. 2021;16:e0258154.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16428",
"author": "S Trapani",
"doi-asserted-by": "publisher",
"first-page": "2509",
"journal-title": "Am J Transpl",
"key": "9269_CR2",
"unstructured": "Trapani S, Masiero L, Puoti F, et al. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: a nationwide population-based study. Am J Transpl. 2021;21:2509–21.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/j.cmi.2022.02.039",
"author": "M Gatti",
"doi-asserted-by": "publisher",
"first-page": "1057",
"journal-title": "Clin Microbiol Infect",
"key": "9269_CR3",
"unstructured": "Gatti M, Rinaldi M, Bussini L, et al. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:1057–65.",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1111/tid.13967",
"author": "AJ Trindade",
"doi-asserted-by": "publisher",
"first-page": "e13967",
"journal-title": "Transpl Infect Dis",
"key": "9269_CR4",
"unstructured": "Trindade AJ, Chapin KC, Gannon WD, et al. Clinical course of SARS-CoV-2 infection and recovery in lung transplant recipients. Transpl Infect Dis. 2022;24:e13967.",
"volume": "24",
"year": "2022"
},
{
"DOI": "10.1111/ajt.16241",
"author": "M Aversa",
"doi-asserted-by": "publisher",
"first-page": "3072",
"journal-title": "Am J Transpl",
"key": "9269_CR5",
"unstructured": "Aversa M, Benvenuto L, Anderson M, et al. COVID-19 in lung transplant recipients: a single center case series from New York City. Am J Transpl. 2020;20:3072–80.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2022.06.011",
"author": "E Roosma",
"doi-asserted-by": "publisher",
"first-page": "1237",
"journal-title": "J Heart Lung Transpl",
"key": "9269_CR6",
"unstructured": "Roosma E, van Gemert JP, de Zwart AES, et al. The effect of COVID-19 on transplant function and development of CLAD in lung transplant patients: a multicenter experience. J Heart Lung Transpl. 2022;41:1237–47.",
"volume": "41",
"year": "2022"
},
{
"DOI": "10.1111/ctr.13511",
"author": "O Manuel",
"doi-asserted-by": "publisher",
"first-page": "e13511",
"journal-title": "Clin Transpl",
"key": "9269_CR7",
"unstructured": "Manuel O, Estabrook M, American Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl. 2019;33:e13511.",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1111/tid.13556",
"author": "M Ringer",
"doi-asserted-by": "publisher",
"first-page": "e13556",
"journal-title": "Transpl Infect Dis",
"key": "9269_CR8",
"unstructured": "Ringer M, Azmy V, Kaman K, et al. A retrospective matched cohort single-center study evaluating outcomes of COVID-19 and the impact of immunomodulation on COVID-19-related cytokine release syndrome in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13556.",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16431",
"author": "RK Avery",
"doi-asserted-by": "publisher",
"first-page": "2498",
"journal-title": "Am J Transpl",
"key": "9269_CR9",
"unstructured": "Avery RK, Chiang TP-Y, Marr KA, et al. Inpatient COVID-19 outcomes in solid organ transplant recipients compared to non-solid organ transplant patients: a retrospective cohort. Am J Transpl. 2021;21:2498–508.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2020.00856",
"author": "G Terrazzano",
"doi-asserted-by": "publisher",
"first-page": "856",
"journal-title": "Front Pharmacol",
"key": "9269_CR10",
"unstructured": "Terrazzano G, Rubino V, Palatucci AT, Giovazzino A, Carriero F, Ruggiero G. An Open question: is it rational to inhibit the mtor-dependent pathway as COVID-19 therapy? Front Pharmacol. 2020;11:856.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104878",
"author": "A Pizzorno",
"doi-asserted-by": "publisher",
"first-page": "104878",
"journal-title": "Antiviral Res",
"key": "9269_CR11",
"unstructured": "Pizzorno A, Padey B, Dubois J, et al. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antiviral Res. 2020;181:104878.",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1111/ctr.14027",
"author": "F Bösch",
"doi-asserted-by": "publisher",
"first-page": "e14027",
"journal-title": "Clin Transpl",
"key": "9269_CR12",
"unstructured": "Bösch F, Börner N, Kemmner S, et al. Attenuated early inflammatory response in solid organ recipients with COVID-19. Clin Transpl. 2020;34:e14027.",
"volume": "34",
"year": "2020"
},
{
"key": "9269_CR13",
"unstructured": "Clinical Spectrum. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 18 August 2023."
},
{
"DOI": "10.1016/j.healun.2019.03.009",
"author": "GM Verleden",
"doi-asserted-by": "publisher",
"first-page": "493",
"journal-title": "J Heart Lung Transpl",
"key": "9269_CR14",
"unstructured": "Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transpl. 2019;38:493–503.",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.3389/fmicb.2021.786233",
"author": "P Colson",
"doi-asserted-by": "publisher",
"first-page": "786233",
"journal-title": "Front Microbiol",
"key": "9269_CR15",
"unstructured": "Colson P, Fournier P-E, Chaudet H, et al. Analysis of SARS-CoV-2 variants from 24,181 patients exemplifies the role of globalization and zoonosis in Pandemics. Front Microbiol. 2021;12:786233.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1002/jmv.29033",
"author": "NN Nguyen",
"doi-asserted-by": "publisher",
"first-page": "e29033",
"journal-title": "J Med Virol",
"key": "9269_CR16",
"unstructured": "Nguyen NN, Houhamdi L, Meddeb L, Colson P, Gautret P. Reinfection with SARS-CoV-2 Omicron BA.4 and BA.5 variants. J Med Virol. 2023;95:e29033.",
"volume": "95",
"year": "2023"
},
{
"key": "9269_CR17",
"unstructured": "Polack FP, Thomas SJ, Kitchin N et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;:NEJMoa2034577."
},
{
"key": "9269_CR18",
"unstructured": "Coronavirus Disease 2019 (COVID-19)| CDC. https://www.cdc.gov/coronavirus/2019-nCoV/index.html. Accessed 18 August 2023."
},
{
"key": "9269_CR19",
"unstructured": "R: The R Project for Statistical Computing. https://www.r-project.org/. Accessed 23 September 2023."
},
{
"DOI": "10.18637/jss.v025.i01",
"doi-asserted-by": "crossref",
"key": "9269_CR20",
"unstructured": "Husson F, Josse J, Lê S. FactoMineR: an R Package for Multivariate Analysis. J Stat Softw 2008; 25."
},
{
"DOI": "10.1111/ajt.16368",
"author": "B Coiffard",
"doi-asserted-by": "publisher",
"first-page": "1586",
"journal-title": "Am J Transpl",
"key": "9269_CR21",
"unstructured": "Coiffard B, Lepper PM, Prud’Homme E, et al. Management of lung transplantation in the COVID-19 era-An international survey. Am J Transpl. 2021;21:1586–96.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16692",
"author": "MR Heldman",
"doi-asserted-by": "publisher",
"first-page": "2774",
"journal-title": "Am J Transpl",
"key": "9269_CR22",
"unstructured": "Heldman MR, Kates OS, Safa K, et al. COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study. Am J Transpl. 2021;21:2774–84.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2022.852973",
"doi-asserted-by": "publisher",
"key": "9269_CR23",
"unstructured": "Pinchera B, Spirito L, Buonomo AR et al. mTOR Inhibitor Use Is Associated With a Favorable Outcome of COVID-19 in Patients of Kidney Transplant: Results of a Retrospective Study. Frontiers in Medicine. 2022; 9. https://www.frontiersin.org/articles/https://doi.org/10.3389/fmed.2022.852973. Accessed 14 July 2022."
},
{
"DOI": "10.1053/j.gastro.2020.11.045",
"author": "LS Belli",
"doi-asserted-by": "publisher",
"first-page": "1151",
"journal-title": "Gastroenterology",
"key": "9269_CR24",
"unstructured": "Belli LS, Fondevila C, Cortesi PA, et al. Protective role of Tacrolimus, Deleterious Role of Age and comorbidities in Liver Transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. 2021;160:1151–e11633.",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1111/tid.13410",
"doi-asserted-by": "crossref",
"key": "9269_CR25",
"unstructured": "Desmazes-Dufeu N, Coltey B, Amari L et al. Discordant courses of COVID-19 in a cohabiting couple of lung transplant recipients. Transpl Infect Dis 2020;:e13410."
},
{
"DOI": "10.1016/j.celrep.2021.108940",
"author": "G Garcia",
"doi-asserted-by": "publisher",
"first-page": "108940",
"journal-title": "Cell Rep",
"key": "9269_CR26",
"unstructured": "Garcia G, Sharma A, Ramaiah A, et al. Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 2021;35:108940.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/cei.12669",
"author": "M Sabbatini",
"doi-asserted-by": "publisher",
"first-page": "230",
"journal-title": "Clin Exp Immunol",
"key": "9269_CR27",
"unstructured": "Sabbatini M, Ruggiero G, Palatucci AT, et al. Oscillatory mTOR inhibition and Treg increase in kidney transplantation. Clin Exp Immunol. 2015;182:230–40.",
"volume": "182",
"year": "2015"
},
{
"DOI": "10.1681/ASN.2020030348",
"author": "M Willicombe",
"doi-asserted-by": "publisher",
"first-page": "1145",
"journal-title": "J Am Soc Nephrol",
"key": "9269_CR28",
"unstructured": "Willicombe M, Thomas D, McAdoo S. COVID-19 and calcineurin inhibitors: should they get left out in the storm? J Am Soc Nephrol. 2020;31:1145–6.",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.virusres.2014.02.010",
"author": "J Carbajo-Lozoya",
"doi-asserted-by": "publisher",
"first-page": "44",
"journal-title": "Virus Res",
"key": "9269_CR29",
"unstructured": "Carbajo-Lozoya J, Ma-Lauer Y, Malešević M, et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014;184:44–53.",
"volume": "184",
"year": "2014"
},
{
"DOI": "10.3389/ti.2022.10369",
"author": "NS Ogando",
"doi-asserted-by": "publisher",
"first-page": "10369",
"journal-title": "Transpl Int",
"key": "9269_CR30",
"unstructured": "Ogando NS, Metscher E, Moes DJAR, et al. The cyclophilin-dependent calcineurin inhibitor voclosporin inhibits SARS-CoV-2 replication in Cell Culture. Transpl Int. 2022;35:10369.",
"volume": "35",
"year": "2022"
},
{
"DOI": "10.1016/j.bcp.2019.03.022",
"author": "AM Liddicoat",
"doi-asserted-by": "publisher",
"first-page": "472",
"journal-title": "Biochem Pharmacol",
"key": "9269_CR31",
"unstructured": "Liddicoat AM, Lavelle EC. Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. 2019;163:472–80.",
"volume": "163",
"year": "2019"
},
{
"DOI": "10.1111/tid.13595",
"author": "A Karruli",
"doi-asserted-by": "publisher",
"first-page": "e13595",
"journal-title": "Transpl Infect Dis",
"key": "9269_CR32",
"unstructured": "Karruli A, Spiezia S, Boccia F, et al. Effect of immunosuppression maintenance in solid organ transplant recipients with COVID-19: systematic review and meta-analysis. Transpl Infect Dis. 2021;23:e13595.",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.10.024",
"author": "M Merli",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "J Infect",
"key": "9269_CR33",
"unstructured": "Merli M, Pasulo L, Perricone G, et al. Impact of immunosuppressive therapy on the severity of COVID-19 in solid organ transplant recipients. J Infect. 2021;82:414–51.",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16807",
"author": "LG Modelli de Andrade",
"doi-asserted-by": "publisher",
"first-page": "610",
"journal-title": "Am J Transpl",
"key": "9269_CR34",
"unstructured": "Modelli de Andrade LG, de Sandes-Freitas TV, Requião-Moura LR, et al. Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients. Am J Transpl. 2022;22:610–25.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1002/iid3.1128",
"author": "UW Choi",
"doi-asserted-by": "publisher",
"first-page": "e1128",
"journal-title": "Immun Inflamm Dis",
"key": "9269_CR35",
"unstructured": "Choi UW, Ai X, Li H, Hao Y, Yao X, Guan Y. Immunosuppressive therapy and COVID-19 infection in patients with NMOSD. Immun Inflamm Dis. 2024;12:e1128.",
"volume": "12",
"year": "2024"
},
{
"DOI": "10.1111/tid.14131",
"doi-asserted-by": "crossref",
"key": "9269_CR36",
"unstructured": "Weder MM, Kadl A. Longitudinal impact of COVID-19 on lung function in lung transplant recipients: time to stop worrying? Transpl Infect Dis 2023;:e14131."
},
{
"DOI": "10.1007/s15010-021-01734-2",
"author": "V Thakur",
"doi-asserted-by": "publisher",
"first-page": "309",
"journal-title": "Infection",
"key": "9269_CR37",
"unstructured": "Thakur V, Bhola S, Thakur P, et al. Waves and variants of SARS-CoV-2: understanding the causes and effect of the COVID-19 catastrophe. Infection. 2022;50:309–25.",
"volume": "50",
"year": "2022"
},
{
"DOI": "10.1007/s11596-021-2395-1",
"author": "L Forchette",
"doi-asserted-by": "publisher",
"first-page": "1037",
"journal-title": "Curr Med Sci",
"key": "9269_CR38",
"unstructured": "Forchette L, Sebastian W, Liu T. A Comprehensive Review of COVID-19 Virology, vaccines, variants, and therapeutics. Curr Med Sci. 2021;41:1037–51.",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1016/j.transproceed.2021.12.014",
"author": "J Zimmermann",
"doi-asserted-by": "publisher",
"first-page": "1504",
"journal-title": "Transpl Proc",
"key": "9269_CR39",
"unstructured": "Zimmermann J, Glueck OM, Fertmann JM, et al. COVID-19 in recent lung transplant recipients: clinical outcomes and management strategies. Transpl Proc. 2022;54:1504–16.",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000003508",
"author": "J Messika",
"doi-asserted-by": "publisher",
"first-page": "177",
"journal-title": "Transplantation",
"key": "9269_CR40",
"unstructured": "Messika J, Eloy P, Roux A, et al. COVID-19 in lung transplant recipients. Transplantation. 2021;105:177–86.",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0257807",
"author": "JC Kamp",
"doi-asserted-by": "publisher",
"first-page": "e0257807",
"journal-title": "PLoS ONE",
"key": "9269_CR41",
"unstructured": "Kamp JC, Hinrichs JB, Fuge J, Ewen R, Gottlieb J. COVID-19 in lung transplant recipients-risk prediction and outcomes. PLoS ONE. 2021;16:e0257807.",
"volume": "16",
"year": "2021"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09269-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Management of immunosuppression in lung transplant recipients and COVID-19 outcomes: an observational retrospective cohort-study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}
