Strategies used for the COVID-OUT decentralized trial of outpatient treatment of SARS-CoV-2
et al., Journal of Clinical and Translational Science, doi:10.1017/cts.2023.668, Nov 2023
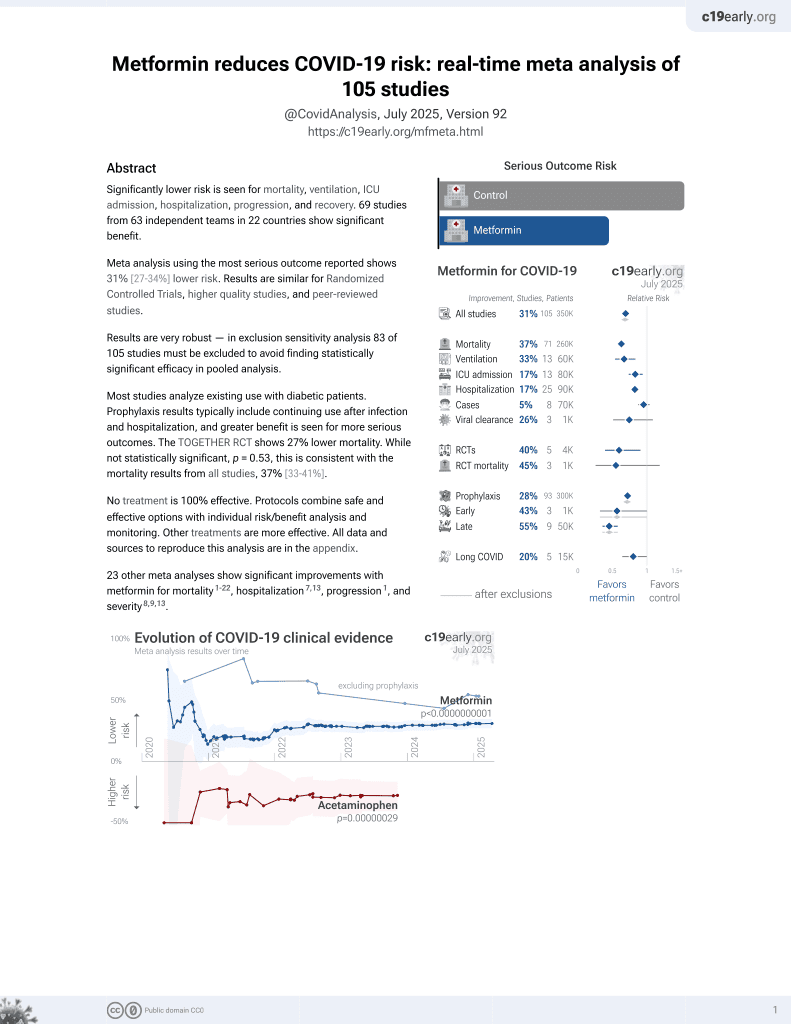
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
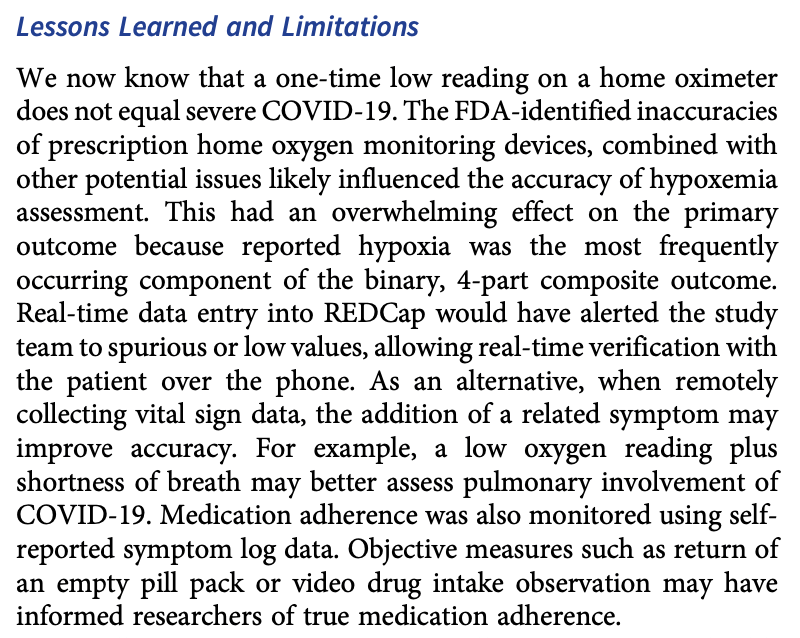
Report on the operation of the COVID-OUT trial noting several issues affecting the reliability of the results:
- Use of home pulse oximeters for measuring oxygen saturation: authors note that the FDA warned about inaccuracies with home pulse oximeters during the trial. A single low reading <94% was part of the composite primary outcome for severe COVID-19. However, home devices can give falsely low readings for various reasons, so basing part of the primary outcome on a single home reading likely introduced significant error.
- Self-reported data: most of the data collected, including symptoms, medication adherence, oxygen levels, etc., was self-reported by participants. Self-reported data is subject to recall bias and other issues that could affect accuracy.
- No objective measurement of medication adherence: the authors note that medication adherence was assessed via self-report in the symptom logs. Without an objective measure like pill counts, actual adherence is uncertain.
- Limited verification of reported medical events/medications: while reported emergency room visits and hospitalizations were verified with records, new medications and medical events outside of participating health systems were not verified, relying only on participant self-report.
- Missing symptom data due to non-returned paper logs: the use of paper symptom logs meant that data was missing if participants did not mail the logs back, hampering data completeness.
- Inaccuracies in sample collection labeling early on: initially some patient samples were missing ID labels, creating challenges for connecting samples to the right patients. This may have led to unusable or mismatched samples.
Review covers ivermectin, metformin, and fluvoxamine.
Avula et al., 7 Nov 2023, peer-reviewed, 21 authors.
Contact: avula010@umn.edu.
Strategies used for the COVID-OUT decentralized trial of outpatient treatment of SARS-CoV-2
Journal of Clinical and Translational Science, doi:10.1017/cts.2023.668
The COVID-19 pandemic accelerated the development of decentralized clinical trials (DCT). DCT's are an important and pragmatic method for assessing health outcomes yet comprise only a minority of clinical trials, and few published methodologies exist. In this report, we detail the operational components of COVID-OUT, a decentralized, multicenter, quadruple-blinded, randomized trial that rapidly delivered study drugs nation-wide. The trial examined three medications (metformin, ivermectin, and fluvoxamine) as outpatient treatment of SARS-CoV-2 for their effectiveness in preventing severe or long COVID-19. Decentralized strategies included HIPAA-compliant electronic screening and consenting, prepacking investigational product to accelerate delivery after randomization, and remotely confirming participantreported outcomes. Of the 1417 individuals with the intention-to-treat sample, the remote nature of the study caused an additional 94 participants to not take any doses of study drug. Therefore, 1323 participants were in the modified intention-to-treat sample, which was the a priori primary study sample. Only 1.4% of participants were lost to follow-up. Decentralized strategies facilitated the successful completion of the COVID-OUT trial without any in-person contact by expediting intervention delivery, expanding trial access geographically, limiting contagion exposure, and making it easy for participants to complete follow-up visits. Remotely completed consent and follow-up facilitated enrollment.
JBB reports contracted fees and travel support for contracted activities for consulting work paid to the University of North Carolina by Novo Nordisk; grant support by Dexcom, NovaTarg, Novo Nordisk, Sanofi, Tolerion and vTv Therapeutics; personal compensation for consultation from Alkahest, Altimmune, Anji, AstraZeneca, Bayer, Biomea Fusion Inc., Boehringer-Ingelheim, CeQur, Cirius Therapeutics Inc., Corcept Therapeutics, Eli Lilly, Fortress Biotech, GentiBio, Glycadia, Glyscend, Janssen, MannKind, Mellitus Health, Moderna, Pendulum Therapeutics, Praetego, Sanofi, Stability Health, Terns Inc., Valo and Zealand Pharma; and stock/options in Glyscend, Mellitus Health, Pendulum Therapeutics, PhaseBio, Praetego, and Stability Health. Dr Puskarich receives consulting fees from Opticyte and Cytovale. The trial was funded by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and the UnitedHealth Group Foundation. Competing interests. The fluvoxamine placebo tablets were donated by the Apotex pharmacy. The ivermectin placebo and active tablets were donated by the Edenbridge pharmacy. The funders had no influence on the design or conduct of the trial and were not involved in data collection or analysis, writing of the manuscript, or decision to submit for publication. The authors assume responsibility for trial fidelity and the accuracy and completeness of the data and analyses.
References
Adepoju, Ojinnaka, Shetty, Angelocci, Utilization gaps during the COVID-19 pandemic: racial and ethnic disparities in Telemedicine Uptake in federally qualified health center clinics, J Gen Intern Med, doi:10.1007/s11606-021-07304-4
Boulware, Pullen, Bangdiwala, A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19, N Engl J Med, doi:10.1056/NEJMoa2016638
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00299-2
Bramante, Huling, Tignanelli, Randomized trial of metformin, ivermectin, and fluvoxamine for covid-19, N Engl J Med, doi:10.1056/NEJMoa2201662
Chiamulera, Mantovani, Tamburin, Remote clinical trials: a timely opportunity for a virtual reality approach and its potential application in neurology, Br J Clin Pharmacol, doi:10.1111/bcp.14922
Dahne, Hawk, Health equity and decentralized trials, JAMA, doi:10.1001/jama.2023.6982
Ely, Ramanan, Kartman, Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00006-6
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Kjerpeseth, Cesta, Furu, Metformin versus insulin and risk of major congenital malformations in pregnancies with Type 2 Diabetes: a nordic register-based cohort study, Diabetes Care, doi:10.2337/dc23-0256
Mitchell, Ahmed, Breeman, It is unprecedented: trial management during the COVID-19 pandemic and beyond, Trials, doi:10.1186/s13063-020-04711-6
Pocock, Stone, The primary outcome fails -what next?, N Engl J Med, doi:10.1056/NEJMra1510064
Schuchat, Walensky, Agency Guidance Review
Simmons, Phipps, Whipps, From hybrid to fully remote clinical trial amidst the COVID-19 pandemic: strategies to promote recruitment, retention, and engagement in a randomized mHealth trial, Digit Health, doi:10.1177/20552076221129065
Skipper, Pastick, Engen, Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial, Ann Intern Med, doi:10.7326/M20-4207
Spiegel, Hays, Bolus, Development of the NIH patientreported outcomes measurement information system (PROMIS) gastrointestinal symptom scales, Am J Gastroenterol, doi:10.1038/ajg.2014.237
Stewart, Krows, Schaafsma, Comparison of racial, ethnic, and geographic location diversity of participants enrolled in clinic-based vs 2 remote COVID-19 clinical trials, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.48325
Tarry-Adkins, Ozanne, Aiken, Impact of metformin treatment during pregnancy on maternal outcomes: a systematic review/metaanalysis, Sci Rep, doi:10.1038/s41598-021-88650-5
Xue, Smietana, Poda, Webster, Yang et al., Clinical trial recovery from COVID-19 disruption, Nat Rev Drug Discov, doi:10.1038/d41573-020-00150-9
DOI record:
{
"DOI": "10.1017/cts.2023.668",
"ISSN": [
"2059-8661"
],
"URL": "http://dx.doi.org/10.1017/cts.2023.668",
"abstract": "<jats:title>Abstract</jats:title>\n\t <jats:p>The COVID-19 pandemic accelerated the development of decentralized clinical trials (DCT). DCT’s are an important and pragmatic method for assessing health outcomes yet comprise only a minority of clinical trials, and few published methodologies exist. In this report, we detail the operational components of COVID-OUT, a decentralized, multicenter, quadruple-blinded, randomized trial that rapidly delivered study drugs nation-wide. The trial examined three medications (metformin, ivermectin, and fluvoxamine) as outpatient treatment of SARS-CoV-2 for their effectiveness in preventing severe or long COVID-19. Decentralized strategies included HIPAA-compliant electronic screening and consenting, prepacking investigational product to accelerate delivery after randomization, and remotely confirming participant-reported outcomes. Of the 1417 individuals with the intention-to-treat sample, the remote nature of the study caused an additional 94 participants to not take any doses of study drug. Therefore, 1323 participants were in the modified intention-to-treat sample, which was the a priori primary study sample. Only 1.4% of participants were lost to follow-up. Decentralized strategies facilitated the successful completion of the COVID-OUT trial without any in-person contact by expediting intervention delivery, expanding trial access geographically, limiting contagion exposure, and making it easy for participants to complete follow-up visits. Remotely completed consent and follow-up facilitated enrollment.</jats:p>",
"alternative-id": [
"S2059866123006684"
],
"article-number": "e242",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5323-2639",
"affiliation": [],
"authenticated-orcid": false,
"family": "Avula",
"given": "Nandini",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kakach",
"given": "Dustin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tignanelli",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liebovitz",
"given": "David M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4418-5919",
"affiliation": [],
"authenticated-orcid": false,
"family": "Nicklas",
"given": "Jacinda M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohen",
"given": "Kenneth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puskarich",
"given": "Michael A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belani",
"given": "Hrishikesh K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9723-3876",
"affiliation": [],
"authenticated-orcid": false,
"family": "Buse",
"given": "John B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klatt",
"given": "Nichole R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anderson",
"given": "Blake",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karger",
"given": "Amy B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hartman",
"given": "Katrina M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Barkha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fenno",
"given": "Sarah L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5327-2876",
"affiliation": [],
"authenticated-orcid": false,
"family": "Reddy",
"given": "Neha V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erickson",
"given": "Spencer M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4715-0060",
"affiliation": [],
"authenticated-orcid": false,
"family": "Boulware",
"given": "David R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murray",
"given": "Thomas A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bramante",
"given": "Carolyn T.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "COVID-OUT Trial Team",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical and Translational Science",
"container-title-short": "J. Clin. Trans. Sci.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
7
]
],
"date-time": "2023-11-07T10:31:10Z",
"timestamp": 1699353070000
},
"deposited": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T02:32:26Z",
"timestamp": 1700706746000
},
"indexed": {
"date-parts": [
[
2023,
11,
23
]
],
"date-time": "2023-11-23T05:20:05Z",
"timestamp": 1700716805138
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 310,
"start": {
"date-parts": [
[
2023,
11,
7
]
],
"date-time": "2023-11-07T00:00:00Z",
"timestamp": 1699315200000
}
}
],
"link": [
{
"URL": "https://www.cambridge.org/core/services/aop-cambridge-core/content/view/S2059866123006684",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "56",
"original-title": [],
"prefix": "10.1017",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
7
]
]
},
"published-print": {
"date-parts": [
[
2023
]
]
},
"publisher": "Cambridge University Press (CUP)",
"reference": [
{
"DOI": "10.1001/jamanetworkopen.2021.48325",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref18"
},
{
"DOI": "10.1038/s41598-021-88650-5",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref9"
},
{
"DOI": "10.1038/d41573-020-00150-9",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref2"
},
{
"DOI": "10.1177/20552076221129065",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref6"
},
{
"author": "Schuchat",
"key": "S2059866123006684_ref16"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref3"
},
{
"DOI": "10.1056/NEJMra1510064",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref14"
},
{
"DOI": "10.2337/dc23-0256",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref8"
},
{
"key": "S2059866123006684_ref10",
"unstructured": "10. ClinCard Participant Support. greenphire.com. https://greenphire.com/participant-payments/clincard-participant-support/. Accessed June 11, 2023."
},
{
"DOI": "10.1007/s11606-021-07304-4",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref19"
},
{
"key": "S2059866123006684_ref11",
"unstructured": "11. Assessing COVID-19-Related Symptoms in Outpatient Adult and Adolescent Subjects in Clinical Trials of Drugs and Biological Products for COVID-19 Prevention or Treatment. Published online September 2020. https://www.fda.gov/media/142143/download. Accessed March 27, 2023."
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref17"
},
{
"DOI": "10.1056/NEJMoa2201662",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref4"
},
{
"DOI": "10.1038/ajg.2014.237",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref12"
},
{
"DOI": "10.1016/S2213-2600(22)00006-6",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref15"
},
{
"DOI": "10.1186/s13063-020-04711-6",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref1"
},
{
"DOI": "10.1056/NEJMoa2016638",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref5"
},
{
"DOI": "10.1111/bcp.14922",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref20"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref7"
},
{
"DOI": "10.1001/jama.2023.6982",
"doi-asserted-by": "publisher",
"key": "S2059866123006684_ref21"
},
{
"key": "S2059866123006684_ref13",
"unstructured": "13. Pulse Oximeter Accuracy and Limitations: FDA Safety Communication. Published online February 19, 2021. https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication. Accessed March 27, 2023."
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cambridge.org/core/product/identifier/S2059866123006684/type/journal_article"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Strategies used for the COVID-OUT decentralized trial of outpatient treatment of SARS-CoV-2",
"type": "journal-article",
"volume": "7"
}
avula