The Role of Inhaled Corticosteroids (ICS) in Critically Ill Patients With COVID-19: A Multicenter, Cohort Study
et al., Journal of Intensive Care Medicine, doi:10.1177/08850666211053548, Nov 2021
Budesonide for COVID-19
28th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
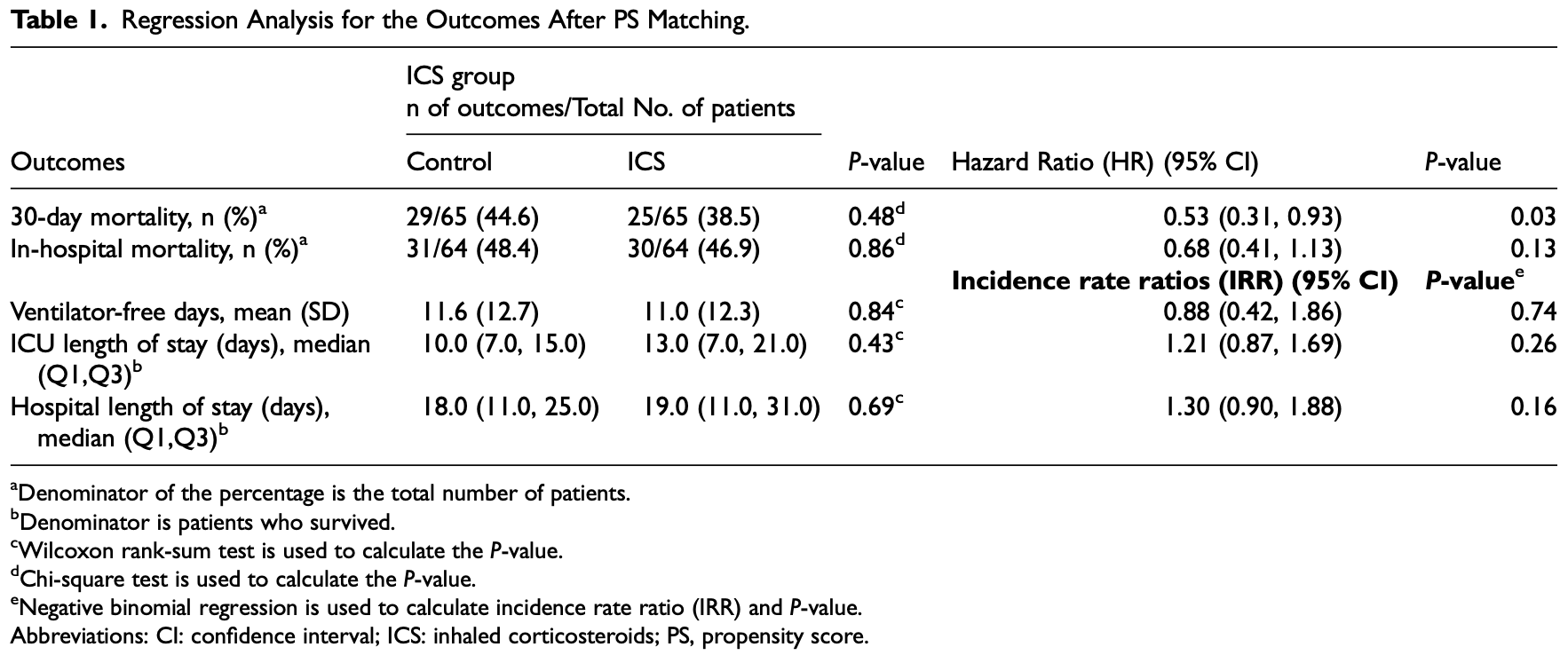
Combined retrospective (Mar-Jun 2020) and prospective (until Mar 2021) study of 954 COVID+ ICU patients in Saudi Arabia, 68 treated with ICS (80% budesonide or budesonide/formoterol, 20% fluticasone/salmeterol), showing lower mortality with treatment, statistically significant for 30-day but not in-hospital mortality.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects (early treatment may be more beneficial).
|
risk of death, 32.0% lower, HR 0.68, p = 0.13, treatment 30 of 64 (46.9%), control 31 of 64 (48.4%), adjusted per study, in-hospital mortality, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of death, 47.0% lower, HR 0.53, p = 0.03, treatment 25 of 65 (38.5%), control 29 of 65 (44.6%), adjusted per study, propensity score matching, multivariable, Cox proportional hazards, day 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Al Sulaiman et al., 10 Nov 2021, prospective, Saudi Arabia, peer-reviewed, 80% of treatment patients used budesonide, mean age 61.4, 24 authors, study period 1 March, 2020 - 31 March, 2021.
Contact: alsulaimankh@hotmail.com.
The Role of Inhaled Corticosteroids (ICS) in Critically Ill Patients With COVID-19: A Multicenter, Cohort Study
Journal of Intensive Care Medicine, doi:10.1177/08850666211053548
Background: Severe coronavirus disease 2019 (COVID-19) can boost the systematic inflammatory response in critically ill patients, causing a systemic hyperinflammatory state leading to multiple complications. In COVID-19 patients, the use of inhaled corticosteroids (ICS) is surrounded by controversy regarding their impacts on viral infections. This study aims to evaluate the safety and efficacy of ICS in critically ill patients with COVID-19 and its clinical outcomes. Method: A multicenter, noninterventional, cohort study for critically ill patients with COVID-19 who received ICS. All patients aged ≥ 18 years old with confirmed COVID-19 and admitted to intensive care units (ICUs) between March 1, 2020 and March 31, 2021 were screened. Eligible patients were classified into two groups based on the use of ICS ± long-acting beta-agonists (LABA) during ICU stay. Propensity score (PS)-matched was used based on patient's Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, systemic corticosteroids use, and acute kidney injury (AKI) within 24 h of ICU admission. We considered a P-value of < 0.05 statistically significant. Results: A total of 954 patients were eligible; 130 patients were included after PS matching (1:1 ratio). The 30-day mortality (hazard ratio [HR] [95% confidence interval [CI]]: 0.53 [0.31, 0.93], P-value = 0.03) was statistically significant lower in patients who received ICS. Conversely, the in-hospital mortality, ventilator-free days (VFDs), ICU length of stay (LOS), and hospital LOS were not statistically significant between the two
Author's Contribution Khalid Al Sulaiman: conceptualization, methodology, software, data curation, writing -0riginal draft preparation, coordination, and management, visualization, investigation. Ohoud Aljuhani: conceptualization, literature review, methodology, investigation, data acquisition and processing, supervision, research coordination, and management, revising and editing the manuscript. Kholoud Al Aamer: data acquisition, data analysis, data processing, data preparation, and original draft writing. Omar Al Shaya: data acquisition, data analysis, data processing, data preparation, interpretation of the data and original draft writing. Abdulrahman Al Shaya: data acquisition, data analysis, data processing, data preparation, interpretation of the data and original draft writing. Alawi S. Alsaeedi: data acquisition, data analysis, data processing, data preparation, interpretation of the data and original draft writing. Alaa Alhubaishi: data curation, writing-original draft, revising and editing the manuscript, visualization, investigation and data interpretation. Ali F. Altebainawi: methodology, software, data curation, revising and editing the manuscript, supervision, visualization, investigation. Alaa Al Harthi: data acquisition, data analysis, data processing, data preparation, and original draft writing. Shorouq Albelwi: data acquisition, data analysis, data processing, data preparation, and original draft writing. Rahaf Almutairi: data acquisition, data analysis,..
References
Ahmad, Rathore, Neurological manifestations and complications of COVID-19: a literature review, J Clin Neurosci Off J Neurosurg Soc Australas, doi:10.1016/j.jocn.2020.05.017
Aleidan, Alkhelaifi, Alsenaid, Incidence and risk factors of carbapenem-resistant enterobacteriaceae infection in intensive care units: a matched case-control study, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2020.1822736
Arentz, Yim, Klaff, Characteristics and outcomes of 21 critically Ill patients With COVID-19 in Washington state, JAMA, doi:10.1001/jama.2020.4326
Azkur, Akdis, Azkur, Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19, Allergy, doi:10.1111/all.14364
Burn, You, Sena, Deep phenotyping of 34,128 adult patients hospitalised with COVID-19 in an international network study, Nat Commun, doi:10.1038/s41467-020-18849-z
Cdc) C For, Dc, People with Certain Medical Conditions
Checchi, Bellini, Bencivenni, Consolo, COVID-19 Dentistry-Related aspects: a literature overview, Int Dent J, doi:10.1111/idj.12601
Choi, Jung, Yoon, Inhaled corticosteroids and COVID-19 risk and mortality: a nationwide cohort study, J Clin Med, doi:10.3390/jcm9113406
Contoli, Pauletti, Rossi, Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD, Eur Respir J, doi:10.1183/13993003.00451-2017
Finney, Glanville, Farne, Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon, J Allergy Clin Immunol, doi:10.1016/j.jaci.2020.09.034
For, Global Strategy for Asthma Management and Prevention
Gold) Gi For, Global Strategy for Prevention, Diagnosis and Management of COPD
Grasselli, Zangrillo, Zanella, Baseline characteristics and outcomes of 1591 patients infected With SARS-CoV-2 admitted to ICUs of the lombardy region, Italy, JAMA, doi:10.1001/jama.2020.5394
Guan, Liang, Zhao, Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J, doi:10.1183/13993003.00547-2020
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Halpin, Faner, Sibila, Badia, Agusti, Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection?, Lancet Respir Med, doi:10.1016/S2213-2600(20)30167-3
Halpin, Singh, Hadfield, Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective, Eur Respir J, doi:10.1183/13993003.01009-2020
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Jeon, Ko, Lee, Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Koenig, Truwit, Ventilator-associated pneumonia: diagnosis, treatment, and prevention, Clin Microbiol Rev, doi:10.1128/CMR.00051-05
Lee, Choi, Jang, Inhaled bronchodilators and the risk of tachyarrhythmias, Int J Cardiol, doi:10.1016/j.ijcard.2015.04.129
Lin, Chen, Acute kidney injury classification: aKIN and RIFLE criteria in critical patients, World J Crit Care Med, doi:10.5492/wjccm.v1.i2.40
Matsuyama, Kawase, Nao, The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol, doi:10.1128/JVI.01648-20
Milne, Li, Yang, Inhaled corticosteroids downregulate SARS-CoV-2-related genes in COPD: results from a randomised controlled trial, Eur Respir J, doi:10.1183/13993003.00130-2021
O'byrne, Pedersen, Carlsson, Risks of pneumonia in patients with asthma taking inhaled corticosteroids, Am J Respir Crit Care Med, doi:10.1164/rccm.201005-0694OC
Pinna, Scabini, Corcione, Lupia, Rosa, COVID-19 pneumonia: do not leave the corticosteroids behind!, Future Microbiol, doi:10.2217/fmb-2020-0199
Ramakrishnan, Nicolau, Langford, Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00160-0
Rodriguez-Roisin, Pulmonary gas exchange in acute respiratory failure, Eur J Anaesthesiol
Schultze, Douglas, COVID-19 and inhaled corticosteroidsanother piece in an expanding puzzle, Lancet Respir Med, doi:10.1016/S2213-2600(21)00076-X
Schultze, Walker, Mackenna, Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform, Lancet Respir Med, doi:10.1016/S2213-2600(20)30415-X
Sen, Majumdar, Zein, Hatipoğlu, Attaway, Inhaled corticosteroids do not adversely impact outcomes in COVID-19 positive patients with COPD: an analysis of Cleveland clinic'S COVID-19 registry, PLoS One, doi:10.1371/journal.pone.0252576
Sheffer, Silverman, Woolcock, Díaz, Lindberg et al., Long-term safety of once-daily budesonide in patients with early-onset mild persistent asthma: results of the inhaled steroid treatment as regular therapy in early asthma (START) study. Ann Allergy, Asthma Immunol Off Publ Am Coll Allergy, Asthma, Immunol, doi:10.1016/S1081-1206(10)61285-9
Singh, Halpin, Inhaled corticosteroids and COVID-19-related mortality: confounding or clarifying?, Lancet Respir Med, doi:10.1016/S2213-2600(20)30447-1
Sulaiman, Aljuhani, Eljaaly, Clinical features and outcomes of critically ill patients with coronavirus disease 2019 (COVID-19): a multicenter cohort study, Int J Infect Dis IJID Off Publ Int Soc Infect Dis, doi:10.1016/j.ijid.2021.02.037
Wang, Liou, Lin, Association of cardiovascular risk With inhaled long-acting bronchodilators in patients With chronic obstructive pulmonary disease: a nested case-control study, JAMA Intern Med, doi:10.1001/jamainternmed.2017.7720
Xie, Wu, Li, Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study, Intensive Care Med, doi:10.1007/s00134-020-06211-2
Yamaya, Nishimura, Deng, Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells, Respir Investig, doi:10.1016/j.resinv.2019.12.005
Yang, Chen, Zhang, Long-term use of inhaled corticosteroids and risk of upper respiratory tract infection in chronic obstructive pulmonary disease: a meta-analysis, Inhal Toxicol, doi:10.1080/08958378.2017.1346006
Yang, Clarke, Sim, Fong, Inhaled corticosteroids for stable chronic obstructive pulmonary disease, Cochrane Database Syst Rev, doi:10.1002/14651858.CD002991.pub3
Yang, Li, Zhang, Sun, Chen, Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis, Crit Care, doi:10.1186/s13054-020-03065-4
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)01744-X
DOI record:
{
"DOI": "10.1177/08850666211053548",
"ISSN": [
"0885-0666",
"1525-1489"
],
"URL": "http://dx.doi.org/10.1177/08850666211053548",
"abstract": "<jats:p> Background: Severe coronavirus disease 2019 (COVID-19) can boost the systematic inflammatory response in critically ill patients, causing a systemic hyperinflammatory state leading to multiple complications. In COVID-19 patients, the use of inhaled corticosteroids (ICS) is surrounded by controversy regarding their impacts on viral infections. This study aims to evaluate the safety and efficacy of ICS in critically ill patients with COVID-19 and its clinical outcomes. Method: A multicenter, noninterventional, cohort study for critically ill patients with COVID-19 who received ICS. All patients aged ≥ 18 years old with confirmed COVID-19 and admitted to intensive care units (ICUs) between March 1, 2020 and March 31, 2021 were screened. Eligible patients were classified into two groups based on the use of ICS ± long-acting beta-agonists (LABA) during ICU stay. Propensity score (PS)-matched was used based on patient’s Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, systemic corticosteroids use, and acute kidney injury (AKI) within 24 h of ICU admission. We considered a P-value of < 0.05 statistically significant. Results: A total of 954 patients were eligible; 130 patients were included after PS matching (1:1 ratio). The 30-day mortality (hazard ratio [HR] [95% confidence interval [CI]]: 0.53 [0.31, 0.93], P-value = 0.03) was statistically significant lower in patients who received ICS. Conversely, the in-hospital mortality, ventilator-free days (VFDs), ICU length of stay (LOS), and hospital LOS were not statistically significant between the two groups. Conclusion: The use of ICS ± LABA in COVID-19 patients may have survival benefits at 30 days. However, it was not associated with in-hospital mortality benefits nor VFDs. </jats:p>",
"alternative-id": [
"10.1177/08850666211053548"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5547-2043",
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Al Sulaiman",
"given": "Khalid",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-8078-564X",
"affiliation": [
{
"name": "Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Aljuhani",
"given": "Ohoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Aamer",
"given": "Kholoud",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2244-0491",
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Al Shaya",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Shaya",
"given": "Abdulrahman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
},
{
"name": "College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Alsaeedi",
"given": "Alawi S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Alhubaishi",
"given": "Alaa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmaceutical Care Services, King Salman Specialist Hospital, Hail Health Cluster, Ministry of Health, Hail, Saudi Arabia"
}
],
"family": "Altebainawi",
"given": "Ali F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Harthi",
"given": "Alaa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Albelwi",
"given": "Shorouq",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Almutairi",
"given": "Rahaf",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
}
],
"family": "Alsubaie",
"given": "Norah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia"
}
],
"family": "Alsallum",
"given": "Alanoud",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2022-5955",
"affiliation": [
{
"name": "College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "Korayem",
"given": "Ghazwa B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia"
}
],
"family": "Alfahed",
"given": "Amjaad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Kensara",
"given": "Raed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Medicine, University of Hail, Hail, Saudi Arabia"
}
],
"family": "Altebainawi",
"given": "Elaf F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Pharmacy, University of Hail, Hail, Saudi Arabia"
}
],
"family": "Alenezi",
"given": "Raghdah S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Alsulaiman",
"given": "Thamer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
}
],
"family": "Al Enazi",
"given": "Huda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Vishwakarma",
"given": "Ramesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Dabbagh",
"given": "Tarek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Bakhsh",
"given": "Umar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Abdulaziz Medical City, Riyadh, Saudi Arabia"
},
{
"name": "King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
},
{
"name": "College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Al Ghamdi",
"given": "Ghassan",
"sequence": "additional"
}
],
"container-title": [
"Journal of Intensive Care Medicine"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
10
]
],
"date-time": "2021-11-10T19:48:54Z",
"timestamp": 1636573734000
},
"deposited": {
"date-parts": [
[
2021,
12,
20
]
],
"date-time": "2021-12-20T14:15:17Z",
"timestamp": 1640009717000
},
"indexed": {
"date-parts": [
[
2022,
2,
11
]
],
"date-time": "2022-02-11T19:48:52Z",
"timestamp": 1644608932015
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0885-0666"
},
{
"type": "electronic",
"value": "1525-1489"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2021,
11,
10
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
10
]
],
"date-time": "2021-11-10T00:00:00Z",
"timestamp": 1636502400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/08850666211053548",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/08850666211053548",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/08850666211053548",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "248-257",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
11,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "bibr1-08850666211053548"
},
{
"DOI": "10.1111/idj.12601",
"doi-asserted-by": "publisher",
"key": "bibr2-08850666211053548"
},
{
"DOI": "10.1007/s00134-020-06211-2",
"doi-asserted-by": "publisher",
"key": "bibr3-08850666211053548"
},
{
"DOI": "10.1001/jama.2020.4326",
"doi-asserted-by": "publisher",
"key": "bibr4-08850666211053548"
},
{
"DOI": "10.1016/j.ijid.2021.02.037",
"doi-asserted-by": "publisher",
"key": "bibr5-08850666211053548"
},
{
"DOI": "10.1016/j.jocn.2020.05.017",
"doi-asserted-by": "publisher",
"key": "bibr6-08850666211053548"
},
{
"DOI": "10.1186/s13054-020-03065-4",
"doi-asserted-by": "publisher",
"key": "bibr7-08850666211053548"
},
{
"DOI": "10.1111/all.14364",
"doi-asserted-by": "publisher",
"key": "bibr8-08850666211053548"
},
{
"DOI": "10.1183/13993003.00547-2020",
"doi-asserted-by": "publisher",
"key": "bibr9-08850666211053548"
},
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "bibr10-08850666211053548"
},
{
"key": "bibr11-08850666211053548",
"unstructured": "(CDC) C for DC and P. People with Certain Medical Conditions. Published 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed March 25, 2021."
},
{
"DOI": "10.1038/s41467-020-18849-z",
"doi-asserted-by": "publisher",
"key": "bibr12-08850666211053548"
},
{
"key": "bibr13-08850666211053548",
"unstructured": "(GINA) GI for A. 2020 Global Strategy for Asthma Management and Prevention. 2020. https://ginasthma.org/gina-reports/"
},
{
"key": "bibr14-08850666211053548",
"unstructured": "(GOLD) GI for COLD. 2021 Global Strategy for Prevention, Diagnosis and Management of COPD. 2021. https://goldcopd.org/2021-gold-reports/"
},
{
"DOI": "10.1080/08958378.2017.1346006",
"doi-asserted-by": "publisher",
"key": "bibr15-08850666211053548"
},
{
"DOI": "10.1002/14651858.CD002991.pub3",
"doi-asserted-by": "publisher",
"key": "bibr16-08850666211053548"
},
{
"DOI": "10.1183/13993003.00451-2017",
"doi-asserted-by": "publisher",
"key": "bibr17-08850666211053548"
},
{
"DOI": "10.1164/rccm.201005-0694OC",
"doi-asserted-by": "publisher",
"key": "bibr18-08850666211053548"
},
{
"DOI": "10.1016/S1081-1206(10)61285-9",
"doi-asserted-by": "publisher",
"key": "bibr19-08850666211053548"
},
{
"DOI": "10.1016/j.jaci.2020.09.034",
"doi-asserted-by": "publisher",
"key": "bibr20-08850666211053548"
},
{
"DOI": "10.1016/S2213-2600(20)30415-X",
"doi-asserted-by": "publisher",
"key": "bibr21-08850666211053548"
},
{
"DOI": "10.1016/S2213-2600(20)30447-1",
"doi-asserted-by": "publisher",
"key": "bibr22-08850666211053548"
},
{
"DOI": "10.1016/j.resinv.2019.12.005",
"doi-asserted-by": "publisher",
"key": "bibr23-08850666211053548"
},
{
"DOI": "10.1128/JVI.01648-20",
"doi-asserted-by": "publisher",
"key": "bibr24-08850666211053548"
},
{
"DOI": "10.1183/13993003.01009-2020",
"doi-asserted-by": "publisher",
"key": "bibr25-08850666211053548"
},
{
"DOI": "10.1016/S2213-2600(20)30167-3",
"doi-asserted-by": "publisher",
"key": "bibr26-08850666211053548"
},
{
"DOI": "10.1128/AAC.00819-20",
"doi-asserted-by": "publisher",
"key": "bibr27-08850666211053548"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "bibr28-08850666211053548"
},
{
"DOI": "10.5492/wjccm.v1.i2.40",
"doi-asserted-by": "publisher",
"key": "bibr29-08850666211053548"
},
{
"author": "Rodriguez-Roisin R",
"first-page": "5",
"issue": "1",
"journal-title": "Eur J Anaesthesiol",
"key": "bibr30-08850666211053548",
"volume": "11",
"year": "1994"
},
{
"author": "ICD-10-CM",
"key": "bibr31-08850666211053548",
"volume-title": "The Complete Official Codebook",
"year": "2016"
},
{
"DOI": "10.1128/CMR.00051-05",
"doi-asserted-by": "publisher",
"key": "bibr32-08850666211053548"
},
{
"DOI": "10.1080/14787210.2020.1822736",
"doi-asserted-by": "publisher",
"key": "bibr33-08850666211053548"
},
{
"DOI": "10.1016/S2213-2600(21)00076-X",
"doi-asserted-by": "publisher",
"key": "bibr34-08850666211053548"
},
{
"key": "bibr35-08850666211053548",
"unstructured": "Protective Role of Inhaled Steroids for Covid-19 Infection (INHASCO). Identifier NCT04331054. https://clinicaltrials.gov/ct2/show/NCT04331054. Accessed July 6, 2021."
},
{
"DOI": "10.1371/journal.pone.0252576",
"doi-asserted-by": "publisher",
"key": "bibr36-08850666211053548"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"doi-asserted-by": "publisher",
"key": "bibr37-08850666211053548"
},
{
"DOI": "10.2217/fmb-2020-0199",
"doi-asserted-by": "publisher",
"key": "bibr38-08850666211053548"
},
{
"DOI": "10.1183/13993003.00130-2021",
"doi-asserted-by": "publisher",
"key": "bibr39-08850666211053548"
},
{
"DOI": "10.3390/jcm9113406",
"doi-asserted-by": "publisher",
"key": "bibr40-08850666211053548"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"doi-asserted-by": "publisher",
"key": "bibr41-08850666211053548"
},
{
"key": "bibr42-08850666211053548",
"unstructured": "Inhaled Corticosteroid Treatment of COVID19 Patients With Pneumonia. Identifier NCT04355637. https://clinicaltrials.gov/ct2/show/NCT04355637. Accessed July 6, 2021."
},
{
"key": "bibr43-08850666211053548",
"unstructured": "Evaluation of Efficacy of Levamisole and Formoterol + Budesonide in Treatment of COVID-19. Identifier NCT04331470. https://clinicaltrials.gov/ct2/show/NCT04331470. Accessed July 6, 2021."
},
{
"key": "bibr44-08850666211053548",
"unstructured": "A Study of the Safety and Efficacy of Ciclesonide in the Treatment of Non-hospitalized COVID-19 Patients. Identifier NCT04377711. https://clinicaltrials.gov/ct2/show/NCT04377711. Accessed July 6, 2021."
},
{
"key": "bibr45-08850666211053548",
"unstructured": "A Trial of Ciclesonide in Adults With Mild-to-moderate COVID-19. Identifier NCT04330586. https://clinicaltrials.gov/ct2/show/NCT04330586. Accessed July 6, 2021."
},
{
"DOI": "10.1001/jamainternmed.2017.7720",
"doi-asserted-by": "publisher",
"key": "bibr46-08850666211053548"
},
{
"DOI": "10.1016/j.ijcard.2015.04.129",
"doi-asserted-by": "publisher",
"key": "bibr47-08850666211053548"
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": null
}
},
"score": 1,
"short-container-title": [
"J Intensive Care Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": [
"The Role of Inhaled Corticosteroids (ICS) in Critically Ill Patients With COVID-19: A Multicenter, Cohort Study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "37"
}
