Efficacy and safety of trimodulin in patients with severe COVID-19: results from a randomised, placebo-controlled, double-blind, multicentre, phase II trial (ESsCOVID)
et al., European Journal of Medical Research, doi:10.1186/s40001-024-02008-x, ESsCOVID, NCT04576728, Aug 2024
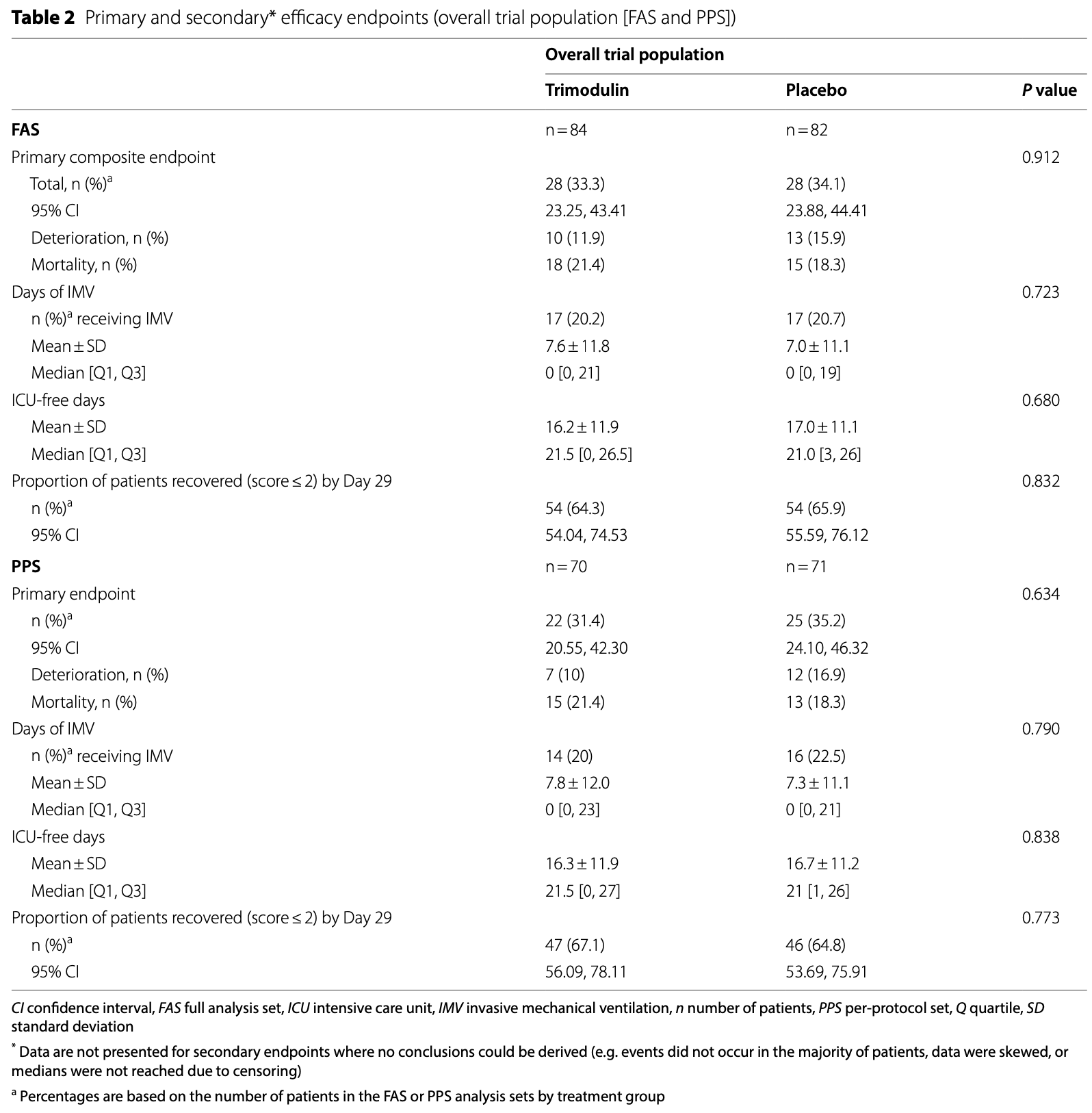
RCT 166 severe COVID-19 patients on non-invasive ventilation or high-flow oxygen showing no significant difference in clinical deterioration or mortality with trimodulin treatment compared to placebo. In a post hoc analysis of a subgroup with early systemic inflammation, excluding patients with CRP > 150 mg/L and/or D-dimer ≥ 3 mg/L and/or platelets < 130 × 109/L at baseline, there was lower clinical deterioration or mortality, without statistical significance in the FAS. Authors hypothesize that trimodulin may reduce inflammation-driven progression if administered earlier before advanced disease develops.
|
risk of death, 17.1% higher, RR 1.17, p = 0.70, treatment 18 of 84 (21.4%), control 15 of 82 (18.3%).
|
|
mortality or deterioration, 2.4% lower, RR 0.98, p = 1.00, treatment 28 of 84 (33.3%), control 28 of 82 (34.1%), NNT 123.
|
|
ventilation time, 8.6% higher, relative time 1.09, p = 0.62, treatment mean 7.6 (±1.18) n=84, control mean 7.0 (±11.1) n=82.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Agafina et al., 13 Aug 2024, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, mean age 58.5, 24 authors, study period 6 October, 2020 - 29 June, 2021, trial NCT04576728 (history) (ESsCOVID).
Contact: atorres@clinic.cat.
Efficacy and safety of trimodulin in patients with severe COVID-19: results from a randomised, placebo-controlled, double-blind, multicentre, phase II trial (ESsCOVID)
European Journal of Medical Research, doi:10.1186/s40001-024-02008-x
Background Trimodulin (human polyvalent immunoglobulin [Ig] M ~ 23%, IgA ~ 21%, IgG ~ 56% preparation) has previously been associated with a lower mortality rate in a subpopulation of patients with severe communityacquired pneumonia on invasive mechanical ventilation (IMV) and with clear signs of inflammation. The hypothesis for the ESsCOVID trial was that trimodulin may prevent inflammation-driven progression of severe coronavirus disease 2019 (COVID-19) to critical disease or even death. Methods Adults with severe COVID-19 were randomised to receive intravenous infusions of trimodulin or placebo for 5 consecutive days in addition to standard of care. The primary efficacy endpoint was a composite of clinical deterioration (Days 6-29) and 28-day all-cause mortality (Days 1-29).
Results One-hundred-and-sixty-six patients received trimodulin (n = 84) or placebo (n = 82). Thirty-three patients died, nine during the treatment phase. Overall, 84.9% and 76.5% of patients completed treatment and follow-up, respectively. The primary efficacy endpoint was reported in 33.3% of patients on trimodulin and 34.1% of patients on placebo (P = 0.912). No differences were observed in the proportion of patients recovered on Day 29, days of invasive mechanical ventilation, or intensive care unit-free days. Rates of treatment-emergent adverse events were comparable. A post hoc analysis was conducted in patients with early systemic inflammation by excluding those with high CRP (> 150 mg/L) and/or D-dimer (≥ 3 mg/L) and/or low platelet counts (< 130 × 10 9 /L) at baseline. Forty-seven patients in the trimodulin group and 49 in the placebo group met these criteria. A difference of 15.5 percentage points
SoC Standard of care SpO 2 Blood oxygen saturation TEEs Thromboembolic events TEAEs Treatment-emergent adverse events TNF Tumour necrosis factor ULN Upper limit of the normal WHO World Health Organization
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s40001-024-02008-x. Additional file 1. Supplementary methods
Additional file 2. Supplementary results
Author contributions
Declarations Ethics approval and consent to participate The trial was conducted according to the International Council for Harmonisation, Good Clinical Practice standards and the Declaration of Helsinki, and with independent ethics committee approval. Written informed consent from the patient, or legally authorised representative, was obtained in compliance with all local legal requirements.
Consent for publication Not applicable.
Competing interests
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bohländer, Riehl, Weißmüller, Gutscher, Schüttrumpf et al., Immunomodulation: immunoglobulin preparations suppress hyperinflammation in a COVID-19 model via FcγRIIA and FcαRI, Front Immunol
Bohländer, Weißmüller, Riehl, Gutscher, Schüttrumpf et al., The functional role of IgA in the IgM/IgA-enriched immunoglobulin preparation trimodulin, Biomedicines
Busani, Roat, Serafini, Mantovani, Biagioni et al., The role of adjunctive therapies in septic shock by Gram negative MDR/XDR infections, Can J Infect Dis Med Microbiol
Busani, Serafini, Mantovani, Venturelli, Giannella et al., Mortality in patients with septic shock by multidrug resistant bacteria, J Intensive Care Med
Camporota, Cronin, Busana, Gattinoni, Formenti, Pathophysiology of coronavirus-19 disease acute lung injury, Curr Opin Crit Care
Cao, Liu, Hong, Ma, Zhang et al., High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China, Front Immunol
Conway, Mackman, Warren, Wolberg, Mosnier et al., Understanding COVID-19-associated coagulopathy, Nat Rev Immunol
Corona, Richini, Simoncini, Zangrandi, Biasini et al., Treating critically ill patients experiencing SARS-CoV-2 severe infection with Ig-M and Ig-A enriched Ig-G infusion, Antibiotics
Dahan, Segal, Katz, Hellou, Tietel et al., Ferritin as a marker of severity in COVID-19 patients: a fatal correlation, Isr Med Assoc J
Dati, Schumann, Thomas, Aguzzi, Baudner et al., Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470), Eur J Clin Chem Clin Biochem
Duerr, Bacher, De Martin, Sachet, Sadeghi et al., The novel polyclonal Ab preparation trimodulin attenuates ex vivo endotoxin-induced immune reactions in early hyperinflammation, Innate Immun
Gharebaghi, Nejadrahim, Mousavi, Sadat-Ebrahimi, Hajizadeh, The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial, BMC Infect Dis
Gold, Wong, Szablewski, Patel, Rossow et al., Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 -Georgia, March 2020, MMWR Morb Mortal Wkly Rep
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, New Engl J Med
Han, Ma, Li, Liu, Zhao et al., Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerg Microbes Infect
Jarczak, Kluge, Nierhaus, Use of intravenous immunoglobulins in sepsis therapy-a clinical view, Int J Mol Sci
Kaushal, Kaur, Sarma, Bhattacharyya, Sharma et al., Serum ferritin as a predictive biomarker in COVID-19. A systematic review, metaanalysis and meta-regression analysis, J Crit Care
Kaveri, Silverman, Bayry, Natural IgM in immune equilibrium and harnessing their therapeutic potential, J Immunol
Lee, Park, Kim, Lee, Kim, Lymphopenia as a biological predictor of outcomes in COVID-19 patients: a nationwide cohort study, Cancers
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat Rev Drug Discov
Li, Hou, Diao, Wang, Yang, Neutrophil-to-lymphocyte ratio is independently associated with COVID-19 severity: an updated meta-analysis based on adjusted effect estimates, Int J Lab Hematol
Li, Li, Xing, Niu, Yao et al., Intravenous immunoglobulin for treatment of hospitalized COVID-19 patients: an evidence mapping and meta-analysis, Inflammopharmacology, doi:10.1007/s10787-023-01398-4
Li, Rong, Cui, Feng, Chen et al., Dynamic changes in serum IL-6, IL-8, and IL-10 predict the outcome of ICU patients with severe COVID-19, Ann Palliat Med
Li, Zhang, Hu, Tong, Zheng et al., Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA
Luo, Zhou, Yan, Guo, Wang et al., Prognostic value of C-reactive protein in patients with coronavirus 2019, Clin Infect Dis
Marcec, Dodig, Radanovic, Likic, Intravenous immunoglobulin (IVIg) therapy in hospitalised adult COVID-19 patients: a systematic review and meta-analysis, Rev Med Virol
Matsuo, Itoh, Kitamura, Kamikubo, Higuchi et al., Intravenous immunoglobulin enhances the killing activity and autophagy of neutrophils isolated from immunocompromised patients against multidrug-resistant bacteria, Biochem Biophys Res Commun
Mohtadi, Ghaysouri, Shirazi, Ansari, Shafiee et al., Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: a case series, Virology
Nierhaus, Berlot, Kindgen-Milles, Müller, Girardis, Bestpractice IgM-and IgA-enriched immunoglobulin use in patients with sepsis, Ann Intensive Care
Notley, Brown, Wright, Ehrenstein, Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells, J Immunol
Obermayer, Afonyushkin, Göderle, Puhm, Schrottmaier et al., Natural IgM antibodies inhibit microvesicle-driven coagulation and thrombosis, Blood
Pan, Yang, Li, Liang, Li et al., Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study, Int J Med Sci
Paules, Wang, Tomashek, Bonnett, Singh et al., A risk profile using simple hematologic parameters to assess benefits from baricitinib in patients hospitalized with COVID-19: a post hoc analysis of the adaptive COVID-19 Treatment trial-2, Ann Intern Med, doi:10.7326/M23-2593
Qin, Zhou, Hu, Zhang, Yang et al., Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China, Clin Infect Dis
Rahmel, Kraft, Haberl, Achtzehn, Brandenburger et al., Intravenous IgM-enriched immunoglobulins in critical COVID-19: a multicentre propensity-weighted cohort study, Crit Care
Ramirez, Romero-Garrido, Lopez-Granados, Borobia, Perez et al., Symptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: results from a retrospective cohort study, Thromb Res
Rieben, Roos, Muizert, Tinguely, Gerritsen et al., Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation, Blood
Robak, Heimesaat, Kruglov, Prepens, Ninnemann et al., Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa Pneumonia, J Clin Invest
Rodriguez, Rello, Neira, Maskin, Ceraso et al., Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery, Shock
Roos, Rieben, Faber-Krol, Daha, IgM-enriched human intravenous immunoglobulin strongly inhibits complement-dependent porcine cell cytotoxicity mediated by human xenoreactive antibodies, Xenotransplantation
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensiv Care Med
Sakoulas, Geriak, Kullar, Greenwood, Habib et al., Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019, Crit Care Explor
Schmidt, Weißmüller, Bohländer, Germer, König et al., The dual role of a polyvalent IgM/IgA-enriched immunoglobulin preparation in activating and inhibiting the complement system, Biomedicines
Schmidt, Weißmüller, Heinz, Multifaceted tissue protective functions of polyvalent immunoglobulin preparations in severe infectionsinteractions with neutrophils, complement, and coagulation pathways, Biomedicines
Shao, Feng, Zhong, Xie, Lei et al., Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: a multicenter retrospective cohort study, Clin Transl Immunol
Singer, Torres, Heinz, Weißmüller, Staus et al., The immunomodulating activity of trimodulin (polyvalent IgM, IgA, IgG solution): a post hoc analysis of the phase II CIGMA trial, Crit Care
Tabarsi, Hashemian, Bauhofer, Savadkoohi, Ghadimi et al., IgM-enriched immunoglobulin in COVID-19: case series of 15 severely ill SARS-CoV-2-infected patients, Int Immunopharmacol
Thachil, Longstaff, Favaloro, Lippi, Urano et al., SSC subcommittee on fibrinolysis of the international society on thrombosis and haemostasis the need for accurate D-dimer reporting in COVID-19: communication from the ISTH SSC on fibrinolysis, J Thromb Haemostat
The, INSIGHT 013) Study group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebocontrolled, phase 3, randomised trial, Lancet
Tomerak, Khan, Almasri, Hussein, Abdelati et al., Systemic inflammation in COVID-19 patients may induce various types of venous and arterial thrombosis: a systematic review, Scand J Immunol
Tziolos, Routsi, Katsenos, Tsangaris, Pneumatikos, Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins, Clin Microbiol Infect
Velavan, Kuk, Linh, Calle, Lalremruata et al., Longitudinal monitoring of laboratory markers characterizes hospitalized and ambulatory COVID-19 patients, Sci Rep
Velavan, Meyer, Mild versus severe COVID-19: laboratory markers, Int J Infect Dis
Welte, Dellinger, Ebelt, Ferrer, Opal et al., Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study), Intensive Care Med
Who, Report of the WHO-China joint mission on coronavirus disease
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention, JAMA
Xiang, Cheng, Li, Luo, Zhang et al., Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis, Int Immunopharmacol
Yang, Liu, Liu, Zhang, Wan et al., COVID-19: immunopathogenesis and immunotherapeutics, Signal Transduct Target Ther
Yuan, Jiao, Qu, Yang, Liu, The development of COVID-19 treatment, Front Immunol
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
Zhu, Zhang, Li, Liu, Zhou et al., Association between thrombocytopenia and 180-day prognosis of COVID-19 patients in intensive care units: a two-center observational study, PLoS ONE
DOI record:
{
"DOI": "10.1186/s40001-024-02008-x",
"ISSN": [
"2047-783X"
],
"URL": "http://dx.doi.org/10.1186/s40001-024-02008-x",
"alternative-id": [
"2008"
],
"article-number": "418",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "15 April 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "1 August 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "13 August 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The trial was conducted according to the International Council for Harmonisation, Good Clinical Practice standards and the Declaration of Helsinki, and with independent ethics committee approval. Written informed consent from the patient, or legally authorised representative, was obtained in compliance with all local legal requirements."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "MSF reports grant support from BioMérieux, speaker fees from BioMérieux and Fisher & Paykel, and consultancy fees from Pfizer (all outside the submitted work); J-FT reports grant support from MSD, Pfizer and Thermo Fisher, consultancy fees from Becton Dickinson, Gilead Sciences, MSD, and Pfizer, speaker fees from MSD, Pfizer and Shionogi, and Chairmanship of the Critical Care section of the European Congress of Clinical Microbiology and Infectious Diseases; JC reports grant support from Biotest and Grifols, and consultancy fees and speaker fees from LFB; SA reports consultancy fees from AstraZeneca and Boehringer Ingelheim, speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis and Sandoz, and support for meeting attendance from AstraZeneca and Boehringer Ingelheim (all outside the submitted work); AT reports consultancy fees and speaker fees from Biotest AG, Janssen, MSD and Pfizer. CCH, TH, PL, IB, AS, MR, and SW are employees of Biotest AG. AW-D was an employee of Biotest AG during trial conduct and the writing of this manuscript. JS is an employee of Grifols SA, as well as an executive board member of Biotest AG, which has received a German Government Grant (Bundesministerium für Bildung und Forschung [BMBF]). AA, VCA, MR, LAH, GT, MF, IG, DP, and RP have no competing interests to declare."
}
],
"author": [
{
"affiliation": [],
"family": "Agafina",
"given": "Alina",
"sequence": "first"
},
{
"affiliation": [],
"family": "Aguiar",
"given": "Valeria Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossovskaya",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fartoukh",
"given": "Muriel Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hajjar",
"given": "Ludhmila Abrahao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thiéry",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Timsit",
"given": "Jean-François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gordeev",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Protsenko",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carbone",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pellegrini",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stadnik",
"given": "Claudio Marcel Berdun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avdeev",
"given": "Sergey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrer",
"given": "Miquel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heinz",
"given": "Corina C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Häder",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langohr",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bobenhausen",
"given": "Iris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schüttrumpf",
"given": "Jörg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Staus",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruehle",
"given": "Markus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weissmüller",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wartenburg-Demand",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres",
"given": "Antoni",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04576728",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "European Journal of Medical Research",
"container-title-short": "Eur J Med Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
8,
13
]
],
"date-time": "2024-08-13T04:02:16Z",
"timestamp": 1723521736000
},
"deposited": {
"date-parts": [
[
2024,
8,
13
]
],
"date-time": "2024-08-13T16:07:33Z",
"timestamp": 1723565253000
},
"funder": [
{
"name": "Biotest AG"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
14
]
],
"date-time": "2024-08-14T00:27:27Z",
"timestamp": 1723595247377
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
8,
13
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
13
]
],
"date-time": "2024-08-13T00:00:00Z",
"timestamp": 1723507200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
13
]
],
"date-time": "2024-08-13T00:00:00Z",
"timestamp": 1723507200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40001-024-02008-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40001-024-02008-x/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40001-024-02008-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
8,
13
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
13
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1097/MCC.0000000000000911",
"author": "L Camporota",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Curr Opin Crit Care",
"key": "2008_CR1",
"unstructured": "Camporota L, Cronin JN, Busana M, Gattinoni L, Formenti F. Pathophysiology of coronavirus-19 disease acute lung injury. Curr Opin Crit Care. 2022;28:9–16.",
"volume": "28",
"year": "2022"
},
{
"key": "2008_CR2",
"unstructured": "WHO. Report of the WHO-China joint mission on coronavirus disease. 2019. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Accessed 29 Jan 2024."
},
{
"key": "2008_CR3",
"unstructured": "National Institutes of Health (NIH). COVID-19 treatment guidelines panel. coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed 29 Jan 2024."
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "publisher",
"first-page": "846",
"journal-title": "Intensiv Care Med",
"key": "2008_CR4",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv Care Med. 2020;46:846–8.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.04.061",
"author": "TP Velavan",
"doi-asserted-by": "publisher",
"first-page": "304",
"journal-title": "Int J Infect Dis",
"key": "2008_CR5",
"unstructured": "Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis. 2020;95:304–7.",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "2008_CR6",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0248671",
"author": "Y Zhu",
"doi-asserted-by": "publisher",
"journal-title": "PLoS ONE",
"key": "2008_CR7",
"unstructured": "Zhu Y, Zhang J, Li Y, Liu F, Zhou Q, Peng Z. Association between thrombocytopenia and 180-day prognosis of COVID-19 patients in intensive care units: a two-center observational study. PLoS ONE. 2021;16: e0248671.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1111/sji.13097",
"author": "S Tomerak",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "Scand J Immunol",
"key": "2008_CR8",
"unstructured": "Tomerak S, Khan S, Almasri M, Hussein R, Abdelati A, Aly A, Salameh MA, Aldien AS, Naveed H, Elshazly MB, Zakaria D. Systemic inflammation in COVID-19 patients may induce various types of venous and arterial thrombosis: a systematic review. Scand J Immunol. 2021;94(5): e13097.",
"volume": "94",
"year": "2021"
},
{
"DOI": "10.1038/s41577-022-00762-9",
"author": "EM Conway",
"doi-asserted-by": "publisher",
"first-page": "639",
"issue": "10",
"journal-title": "Nat Rev Immunol",
"key": "2008_CR9",
"unstructured": "Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, Gralinski LE, Rondina MT, van de Veerdonk FL, Hoffmeister KM, Griffin JH, Nugent D, Moon K, Morrissey JH. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22(10):639–49.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2023.1125246",
"author": "Y Yuan",
"doi-asserted-by": "publisher",
"first-page": "1125246",
"journal-title": "Front Immunol",
"key": "2008_CR10",
"unstructured": "Yuan Y, Jiao B, Qu L, Yang D, Liu R. The development of COVID-19 treatment. Front Immunol. 2023;14:1125246.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"author": "G Li",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Nat Rev Drug Discov",
"key": "2008_CR11",
"unstructured": "Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023;22:449–75.",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.7326/M23-2593",
"author": "CI Paules",
"doi-asserted-by": "publisher",
"journal-title": "Ann Intern Med",
"key": "2008_CR12",
"unstructured": "Paules CI, Wang J, Tomashek KM, Bonnett T, Singh K, Marconi VC, Davey RT Jr, Lye DC, Dodd LE, Yang OO, Benson CA, Deye GA, Doernberg SB, Hynes NA, Grossberg R, Wolfe CR, Nayak SU, Short WR, Voell J, Potter GE, Rapaka RR. A risk profile using simple hematologic parameters to assess benefits from baricitinib in patients hospitalized with COVID-19: a post hoc analysis of the adaptive COVID-19 Treatment trial-2. Ann Intern Med. 2024. https://doi.org/10.7326/M23-2593.",
"year": "2024"
},
{
"DOI": "10.3390/biomedicines9070817",
"author": "C Schmidt",
"doi-asserted-by": "publisher",
"first-page": "817",
"journal-title": "Biomedicines",
"key": "2008_CR13",
"unstructured": "Schmidt C, Weißmüller S, Bohländer F, Germer M, König M, Staus A, Wartenberg-Demand A, Heinz CC, Schüttrumpf J. The dual role of a polyvalent IgM/IgA-enriched immunoglobulin preparation in activating and inhibiting the complement system. Biomedicines. 2021;9:817.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1182/blood.V93.3.942",
"author": "R Rieben",
"doi-asserted-by": "publisher",
"first-page": "942",
"journal-title": "Blood",
"key": "2008_CR14",
"unstructured": "Rieben R, Roos A, Muizert Y, Tinguely C, Gerritsen AF, Daha MR. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood. 1999;93:942–51.",
"volume": "93",
"year": "1999"
},
{
"DOI": "10.1034/j.1399-3089.2003.00063.x",
"author": "A Roos",
"doi-asserted-by": "publisher",
"first-page": "596",
"journal-title": "Xenotransplantation",
"key": "2008_CR15",
"unstructured": "Roos A, Rieben R, Faber-Krol MC, Daha MR. IgM-enriched human intravenous immunoglobulin strongly inhibits complement-dependent porcine cell cytotoxicity mediated by human xenoreactive antibodies. Xenotransplantation. 2003;10:596–605.",
"volume": "10",
"year": "2003"
},
{
"DOI": "10.1177/1753425919853333",
"author": "C Duerr",
"doi-asserted-by": "publisher",
"first-page": "374",
"journal-title": "Innate Immun",
"key": "2008_CR16",
"unstructured": "Duerr C, Bacher A, de Martin A, Sachet M, Sadeghi K, Baumann S, Heinz C, Spittler A. The novel polyclonal Ab preparation trimodulin attenuates ex vivo endotoxin-induced immune reactions in early hyperinflammation. Innate Immun. 2019;25:374–88.",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.3389/fimmu.2021.700429",
"author": "F Bohländer",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "2008_CR17",
"unstructured": "Bohländer F, Riehl D, Weißmüller S, Gutscher M, Schüttrumpf J, Faust S. Immunomodulation: immunoglobulin preparations suppress hyperinflammation in a COVID-19 model via FcγRIIA and FcαRI. Front Immunol. 2021;12: 700429.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3390/biomedicines9121828",
"author": "F Bohländer",
"doi-asserted-by": "publisher",
"first-page": "1828",
"journal-title": "Biomedicines",
"key": "2008_CR18",
"unstructured": "Bohländer F, Weißmüller S, Riehl D, Gutscher M, Schüttrumpf J, Faust S. The functional role of IgA in the IgM/IgA-enriched immunoglobulin preparation trimodulin. Biomedicines. 2021;9:1828.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.4049/jimmunol.1102107",
"author": "SV Kaveri",
"doi-asserted-by": "publisher",
"first-page": "939",
"journal-title": "J Immunol",
"key": "2008_CR19",
"unstructured": "Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol. 2012;188:939–45.",
"volume": "188",
"year": "2012"
},
{
"DOI": "10.4049/jimmunol.1003021",
"author": "CA Notley",
"doi-asserted-by": "publisher",
"first-page": "4967",
"journal-title": "J Immunol",
"key": "2008_CR20",
"unstructured": "Notley CA, Brown MA, Wright GP, Ehrenstein MR. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol. 2011;186:4967–72.",
"volume": "186",
"year": "2011"
},
{
"DOI": "10.1182/blood.2020007155",
"author": "G Obermayer",
"doi-asserted-by": "publisher",
"first-page": "1406",
"journal-title": "Blood",
"key": "2008_CR21",
"unstructured": "Obermayer G, Afonyushkin T, Göderle L, Puhm F, Schrottmaier W, Taqi S, Schwameis M, Ay C, Pabinger I, Jilma B, Assinger A, Mackman N, Binder CJ. Natural IgM antibodies inhibit microvesicle-driven coagulation and thrombosis. Blood. 2021;137:1406–15.",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.1007/s00134-018-5143-7",
"author": "T Welte",
"doi-asserted-by": "publisher",
"first-page": "438",
"journal-title": "Intensive Care Med",
"key": "2008_CR22",
"unstructured": "Welte T, Dellinger RP, Ebelt H, Ferrer M, Opal SM, Singer M, et al. Efficacy and safety of trimodulin, a novel polyclonal antibody preparation, in patients with severe community-acquired pneumonia: a randomized, placebo-controlled, double-blind, multicenter, phase II trial (CIGMA study). Intensive Care Med. 2018;44:438–48.",
"volume": "44",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "W Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "New Engl J Med",
"key": "2008_CR23",
"unstructured": "Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382:1708–20.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"author": "Z Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"journal-title": "JAMA",
"key": "2008_CR24",
"unstructured": "Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–42.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6918e1",
"author": "JAW Gold",
"doi-asserted-by": "publisher",
"first-page": "545",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2008_CR25",
"unstructured": "Gold JAW, Wong KK, Szablewski CM, Patel PR, Rossow J, da Silva J, et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 — Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545–50.",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"author": "L Li",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "JAMA",
"key": "2008_CR26",
"unstructured": "Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:1–11.",
"volume": "324",
"year": "2020"
},
{
"key": "2008_CR27",
"unstructured": "MedDRA version 17.1. https://www.meddra.org/how-to-use/support-documentation/english. Accessed 9 Jan 2024."
},
{
"DOI": "10.1038/s41598-021-93950-x",
"author": "TP Velavan",
"doi-asserted-by": "publisher",
"first-page": "14471",
"journal-title": "Sci Rep",
"key": "2008_CR28",
"unstructured": "Velavan TP, Kuk S, Linh LTK, Calle CL, Lalremruata A, Pallerla SR, et al. Longitudinal monitoring of laboratory markers characterizes hospitalized and ambulatory COVID-19 patients. Sci Rep. 2021;11:14471.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa641",
"author": "X Luo",
"doi-asserted-by": "publisher",
"first-page": "2174",
"journal-title": "Clin Infect Dis",
"key": "2008_CR29",
"unstructured": "Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, Ye L, Xiong J, Jiang Z, Liu Y, Zhang B, Yang W. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71:2174–9.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.7150/ijms.46614",
"author": "F Pan",
"doi-asserted-by": "publisher",
"first-page": "1281",
"journal-title": "Int J Med Sci",
"key": "2008_CR30",
"unstructured": "Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): a case-control study. Int J Med Sci. 2020;17:1281–92.",
"volume": "17",
"year": "2020"
},
{
"author": "F Dati",
"first-page": "517",
"journal-title": "Eur J Clin Chem Clin Biochem",
"key": "2008_CR31",
"unstructured": "Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Petersen PH, Johnson AM, Milford-Ward A, Ritchie RF, Svendsen PJ, Whicher J. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470). Eur J Clin Chem Clin Biochem. 1996;34:517–20.",
"volume": "34",
"year": "1996"
},
{
"DOI": "10.3390/biomedicines11113022",
"author": "C Schmidt",
"doi-asserted-by": "publisher",
"first-page": "3022",
"journal-title": "Biomedicines",
"key": "2008_CR32",
"unstructured": "Schmidt C, Weißmüller S, Heinz CC. Multifaceted tissue protective functions of polyvalent immunoglobulin preparations in severe infections - interactions with neutrophils, complement, and coagulation pathways. Biomedicines. 2023;11:3022.",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1186/s13054-023-04719-9",
"author": "M Singer",
"doi-asserted-by": "publisher",
"first-page": "436",
"journal-title": "Crit Care",
"key": "2008_CR33",
"unstructured": "Singer M, Torres A, Heinz CC, Weißmüller S, Staus A, Kistner S, Jakubczyk K, Häder T, Langohr P, Wartenberg-Demand A, Schüttrumpf J, Vincent J-L, Welte T. The immunomodulating activity of trimodulin (polyvalent IgM, IgA, IgG solution): a post hoc analysis of the phase II CIGMA trial. Crit Care. 2023;27:436.",
"volume": "27",
"year": "2023"
},
{
"DOI": "10.1177/0885066616688165",
"author": "S Busani",
"doi-asserted-by": "publisher",
"first-page": "48",
"journal-title": "J Intensive Care Med",
"key": "2008_CR34",
"unstructured": "Busani S, Serafini G, Mantovani E, Venturelli C, Giannella M, Viale P, et al. Mortality in patients with septic shock by multidrug resistant bacteria. J Intensive Care Med. 2019;34:48–54.",
"volume": "34",
"year": "2019"
},
{
"DOI": "10.1016/j.cmi.2016.01.021",
"author": "EJ Giamarellos-Bourboulis",
"doi-asserted-by": "publisher",
"first-page": "499",
"journal-title": "Clin Microbiol Infect",
"key": "2008_CR35",
"unstructured": "Giamarellos-Bourboulis EJ, Tziolos N, Routsi C, Katsenos C, Tsangaris I, Pneumatikos I, et al. Improving outcomes of severe infections by multidrug-resistant pathogens with polyclonal IgM-enriched immunoglobulins. Clin Microbiol Infect. 2016;22:499–506.",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1155/2017/2808203",
"author": "S Busani",
"doi-asserted-by": "publisher",
"first-page": "2808203",
"journal-title": "Can J Infect Dis Med Microbiol",
"key": "2008_CR36",
"unstructured": "Busani S, Roat E, Serafini G, Mantovani E, Biagioni E, Girardis M. The role of adjunctive therapies in septic shock by Gram negative MDR/XDR infections. Can J Infect Dis Med Microbiol. 2017;2017:2808203.",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1172/JCI97065",
"author": "OH Robak",
"doi-asserted-by": "publisher",
"first-page": "3535",
"journal-title": "J Clin Invest",
"key": "2008_CR37",
"unstructured": "Robak OH, Heimesaat MM, Kruglov AA, Prepens S, Ninnemann J, Gutbier B, et al. Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa Pneumonia. J Clin Invest. 2018;128:3535–45.",
"volume": "128",
"year": "2018"
},
{
"DOI": "10.1016/j.bbrc.2015.06.004",
"author": "H Matsuo",
"doi-asserted-by": "publisher",
"first-page": "94",
"journal-title": "Biochem Biophys Res Commun",
"key": "2008_CR38",
"unstructured": "Matsuo H, Itoh H, Kitamura N, Kamikubo Y, Higuchi T, Shiga S, et al. Intravenous immunoglobulin enhances the killing activity and autophagy of neutrophils isolated from immunocompromised patients against multidrug-resistant bacteria. Biochem Biophys Res Commun. 2015;464:94–9.",
"volume": "464",
"year": "2015"
},
{
"DOI": "10.1097/01.shk.0000157302.69125.f8",
"author": "A Rodriguez",
"doi-asserted-by": "publisher",
"first-page": "298",
"journal-title": "Shock",
"key": "2008_CR39",
"unstructured": "Rodriguez A, Rello J, Neira J, Maskin B, Ceraso D, Vasta L, Palizas F. Effects of high-dose of intravenous immunoglobulin and antibiotics on survival for severe sepsis undergoing surgery. Shock. 2005;23:298–304.",
"volume": "23",
"year": "2005"
},
{
"DOI": "10.1186/s13613-020-00740-1",
"author": "A Nierhaus",
"doi-asserted-by": "publisher",
"first-page": "132",
"journal-title": "Ann Intensive Care",
"key": "2008_CR40",
"unstructured": "Nierhaus A, Berlot G, Kindgen-Milles D, Müller E, Girardis M. Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis. Ann Intensive Care. 2020;10:132.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.3390/ijms21155543",
"author": "D Jarczak",
"doi-asserted-by": "publisher",
"first-page": "5543",
"journal-title": "Int J Mol Sci",
"key": "2008_CR41",
"unstructured": "Jarczak D, Kluge S, Nierhaus A. Use of intravenous immunoglobulins in sepsis therapy—a clinical view. Int J Mol Sci. 2020;21:5543.",
"volume": "21",
"year": "2020"
},
{
"author": "S Dahan",
"first-page": "494",
"journal-title": "Isr Med Assoc J",
"key": "2008_CR42",
"unstructured": "Dahan S, Segal G, Katz I, Hellou T, Tietel M, Bryk G, Amital H, Shoenfeld Y, Dagan A. Ferritin as a marker of severity in COVID-19 patients: a fatal correlation. Isr Med Assoc J. 2020;22:494–500.",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2021.09.023",
"author": "K Kaushal",
"doi-asserted-by": "publisher",
"first-page": "172",
"journal-title": "J Crit Care",
"key": "2008_CR43",
"unstructured": "Kaushal K, Kaur H, Sarma P, Bhattacharyya A, Sharma DJ, Prajapat M, Pathak M, Kothari A, Kumar S, Rana S, Kaur M, Prakash A, Mirza AA, Panda PK, Vivekanandan S, Omar BJ, Medhi B, Naithani M. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care. 2022;67:172–81.",
"volume": "67",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"author": "H Han",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "Emerg Microbes Infect",
"key": "2008_CR44",
"unstructured": "Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123–30.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1038/s41392-020-00243-2",
"author": "L Yang",
"doi-asserted-by": "publisher",
"first-page": "128",
"journal-title": "Signal Transduct Target Ther",
"key": "2008_CR45",
"unstructured": "Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct Target Ther. 2020;5:128.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.21037/apm-20-2134",
"author": "J Li",
"doi-asserted-by": "publisher",
"first-page": "3706",
"journal-title": "Ann Palliat Med",
"key": "2008_CR46",
"unstructured": "Li J, Rong L, Cui R, Feng J, Jin Y, Chen X, Xu R. Dynamic changes in serum IL-6, IL-8, and IL-10 predict the outcome of ICU patients with severe COVID-19. Ann Palliat Med. 2021;10:3706–14.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1111/ijlh.13475",
"author": "Y Li",
"doi-asserted-by": "publisher",
"first-page": "e254",
"journal-title": "Int J Lab Hematol",
"key": "2008_CR47",
"unstructured": "Li Y, Hou H, Diao J, Wang J, Yang H. Neutrophil-to-lymphocyte ratio is independently associated with COVID-19 severity: an updated meta-analysis based on adjusted effect estimates. Int J Lab Hematol. 2021;43:e254–60.",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1016/j.thromres.2014.03.046",
"author": "E Ramirez",
"doi-asserted-by": "publisher",
"first-page": "1045",
"journal-title": "Thromb Res",
"key": "2008_CR48",
"unstructured": "Ramirez E, Romero-Garrido JA, Lopez-Granados E, Borobia AM, Perez T, Medrano N, Rueda C, Tong HY, Herrero A, Frías J. Symptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: results from a retrospective cohort study. Thromb Res. 2014;133:1045–51.",
"volume": "133",
"year": "2014"
},
{
"DOI": "10.3390/cancers13030471",
"author": "J Lee",
"doi-asserted-by": "publisher",
"first-page": "471",
"journal-title": "Cancers (Basel)",
"key": "2008_CR49",
"unstructured": "Lee J, Park SS, Kim TY, Lee DG, Kim DW. Lymphopenia as a biological predictor of outcomes in COVID-19 patients: a nationwide cohort study. Cancers (Basel). 2021;13:471.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa248",
"author": "C Qin",
"doi-asserted-by": "publisher",
"first-page": "762",
"journal-title": "China Clin Infect Dis",
"key": "2008_CR50",
"unstructured": "Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–8.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.3390/antibiotics10080930",
"author": "A Corona",
"doi-asserted-by": "publisher",
"first-page": "930",
"journal-title": "Antibiotics",
"key": "2008_CR51",
"unstructured": "Corona A, Richini G, Simoncini S, Zangrandi M, Biasini M, Russo G, Pasqua M, Santorsola C, Gregorini C, Giordano C. Treating critically ill patients experiencing SARS-CoV-2 severe infection with Ig-M and Ig-A enriched Ig-G infusion. Antibiotics. 2021;10:930.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2021.107998",
"author": "P Tabarsi",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "2008_CR52",
"unstructured": "Tabarsi P, Hashemian SMR, Bauhofer A, Savadkoohi AA, Ghadimi S, Haseli S, Dastan F. IgM-enriched immunoglobulin in COVID-19: case series of 15 severely ill SARS-CoV-2-infected patients. Int Immunopharmacol. 2021;99: 107998.",
"volume": "99",
"year": "2021"
},
{
"DOI": "10.1186/s13054-022-04059-0",
"author": "T Rahmel",
"doi-asserted-by": "publisher",
"first-page": "204",
"journal-title": "Crit Care",
"key": "2008_CR53",
"unstructured": "Rahmel T, Kraft F, Haberl H, Achtzehn U, Brandenburger T, Neb H, et al. Intravenous IgM-enriched immunoglobulins in critical COVID-19: a multicentre propensity-weighted cohort study. Crit Care. 2022;26:204.",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1002/cti2.1192",
"author": "Z Shao",
"doi-asserted-by": "publisher",
"journal-title": "Clin Transl Immunol",
"key": "2008_CR54",
"unstructured": "Shao Z, Feng Y, Zhong L, Xie Q, Lei M, Liu Z, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunol. 2020;9: e1192.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1097/CCE.0000000000000280",
"author": "G Sakoulas",
"doi-asserted-by": "publisher",
"journal-title": "Crit Care Explor",
"key": "2008_CR55",
"unstructured": "Sakoulas G, Geriak M, Kullar R, Greenwood KL, Habib M, Vyas A, Ghafourian M, Dintyala VNK, Haddad F. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2: e0280.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1186/s12879-020-05507-4",
"author": "N Gharebaghi",
"doi-asserted-by": "publisher",
"first-page": "786",
"journal-title": "BMC Infect Dis",
"key": "2008_CR56",
"unstructured": "Gharebaghi N, Nejadrahim R, Mousavi SJ, Sadat-Ebrahimi S-R, Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20:786.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.virol.2020.05.006",
"author": "N Mohtadi",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Virology",
"key": "2008_CR57",
"unstructured": "Mohtadi N, Ghaysouri A, Shirazi S, Ansari S, Shafiee E, Bastani E, Kokhazadeh T, Tavan H. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: a case series. Virology. 2020;548:1–5.",
"volume": "548",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.627844",
"author": "W Cao",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "2008_CR58",
"unstructured": "Cao W, Liu X, Hong K, Ma Z, Zhang Y, Lin L, Han Y, Xiong Y, Liu Z, Ruan L, Li T. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front Immunol. 2021;12: 627844.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)00101-5",
"author": "The ITAC (INSIGHT 013) Study group",
"doi-asserted-by": "publisher",
"first-page": "530",
"journal-title": "Lancet",
"key": "2008_CR59",
"unstructured": "The ITAC (INSIGHT 013) Study group. Hyperimmune immunoglobulin for hospitalised patients with COVID-19 (ITAC): a double-blind, placebo-controlled, phase 3, randomised trial. Lancet. 2022;399:530–40.",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.intimp.2021.107732",
"author": "H-R Xiang",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "2008_CR60",
"unstructured": "Xiang H-R, Cheng X, Li Y, Luo W-W, Zhang Q-Z, Peng W-X. Efficacy of IVIG (intravenous immunoglobulin) for corona virus disease 2019 (COVID-19): a meta-analysis. Int Immunopharmacol. 2021;96: 107732.",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1007/s10787-023-01398-4",
"author": "MX Li",
"doi-asserted-by": "publisher",
"journal-title": "Inflammopharmacology",
"key": "2008_CR61",
"unstructured": "Li MX, Li YF, Xing X, Niu JQ, Yao L, Lu MY, Guo K, Ma MN, Wu XT, Ma N, Li D, Li ZJ, Guan L, Wang XM, Pan B, Shang WR, Ji J, Song ZY, Zhang ZM, Wang YF, Yang KH. Intravenous immunoglobulin for treatment of hospitalized COVID-19 patients: an evidence mapping and meta-analysis. Inflammopharmacology. 2023. https://doi.org/10.1007/s10787-023-01398-4.",
"year": "2023"
},
{
"DOI": "10.1002/rmv.2397",
"author": "R Marcec",
"doi-asserted-by": "publisher",
"journal-title": "Rev Med Virol",
"key": "2008_CR62",
"unstructured": "Marcec R, Dodig VM, Radanovic I, Likic R. Intravenous immunoglobulin (IVIg) therapy in hospitalised adult COVID-19 patients: a systematic review and meta-analysis. Rev Med Virol. 2022;12: e2397.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1111/jth.14956",
"author": "J Thachil",
"doi-asserted-by": "publisher",
"first-page": "2408",
"journal-title": "J Thromb Haemostat.",
"key": "2008_CR63",
"unstructured": "Thachil J, Longstaff C, Favaloro EJ, Lippi G, Urano T, Kim PY. SSC subcommittee on fibrinolysis of the international society on thrombosis and haemostasis the need for accurate D-dimer reporting in COVID-19: communication from the ISTH SSC on fibrinolysis. J Thromb Haemostat. 2020;18:2408–11.",
"volume": "18",
"year": "2020"
}
],
"reference-count": 63,
"references-count": 63,
"relation": {},
"resource": {
"primary": {
"URL": "https://eurjmedres.biomedcentral.com/articles/10.1186/s40001-024-02008-x"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of trimodulin in patients with severe COVID-19: results from a randomised, placebo-controlled, double-blind, multicentre, phase II trial (ESsCOVID)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "29"
}