Aug 13 2024 |
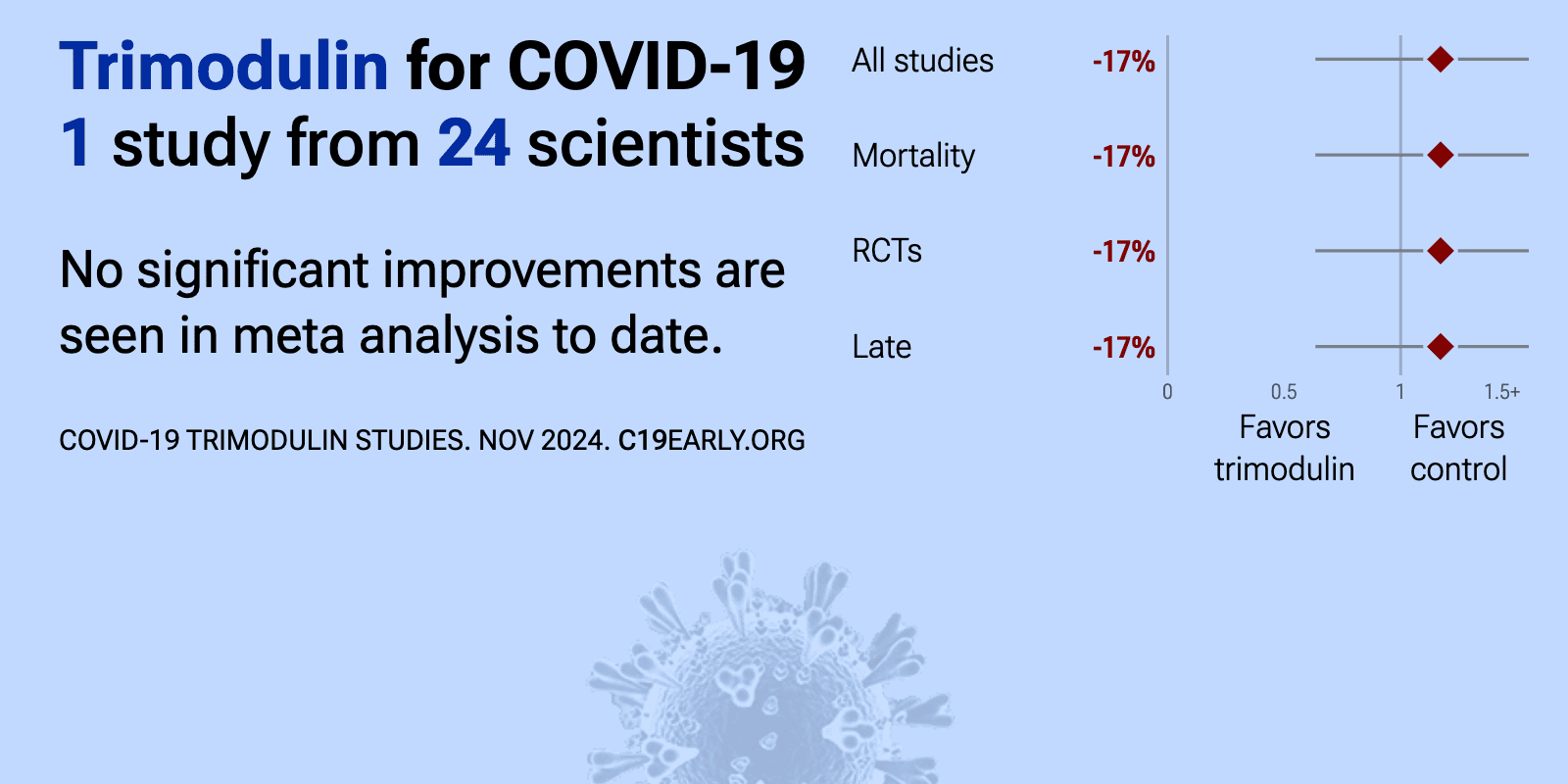
et al., European Journal of Medical Research, doi:10.1186/s40001-024-02008-x | Efficacy and safety of trimodulin in patients with severe COVID-19: results from a randomised, placebo-controlled, double-blind, multicentre, phase II trial (ESsCOVID) |
| 17% higher mortality (p=0.7), 2% lower progression (p=1), and 9% longer ventilation (p=0.62). RCT 166 severe COVID-19 patients on non-invasive ventilation or high-flow oxygen showing no significant difference in clinical deterioration or mortality with trimodulin treatment compared to placebo. In a post hoc analysis of a subgroup .. | ||