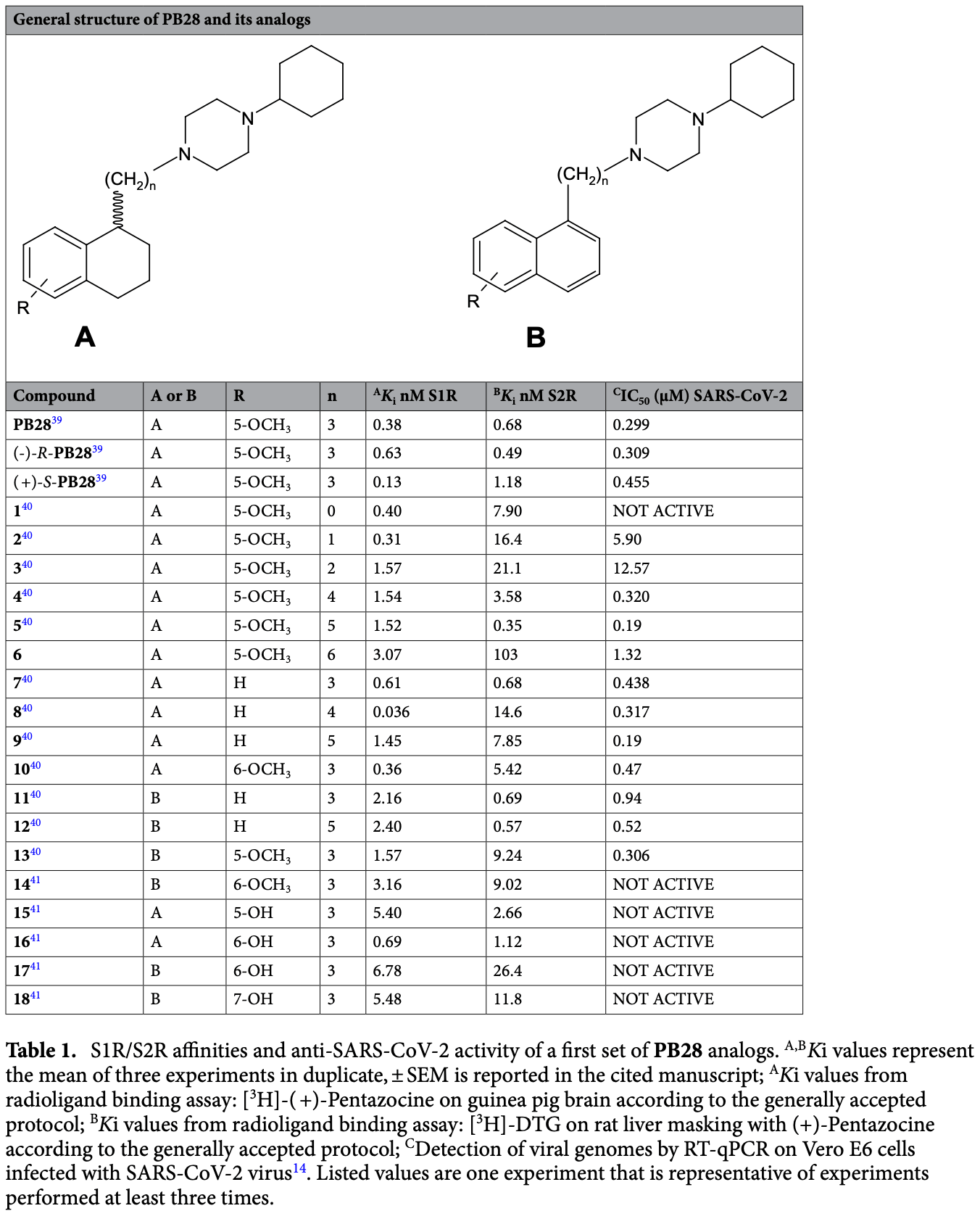
A conformational rearrangement of the SARS-CoV-2 host protein sigma-1 is required for antiviral activity: insights from a combined in-silico/in-vitro approach
et al., Scientific Reports, doi:10.1038/s41598-023-39662-w, Aug 2023
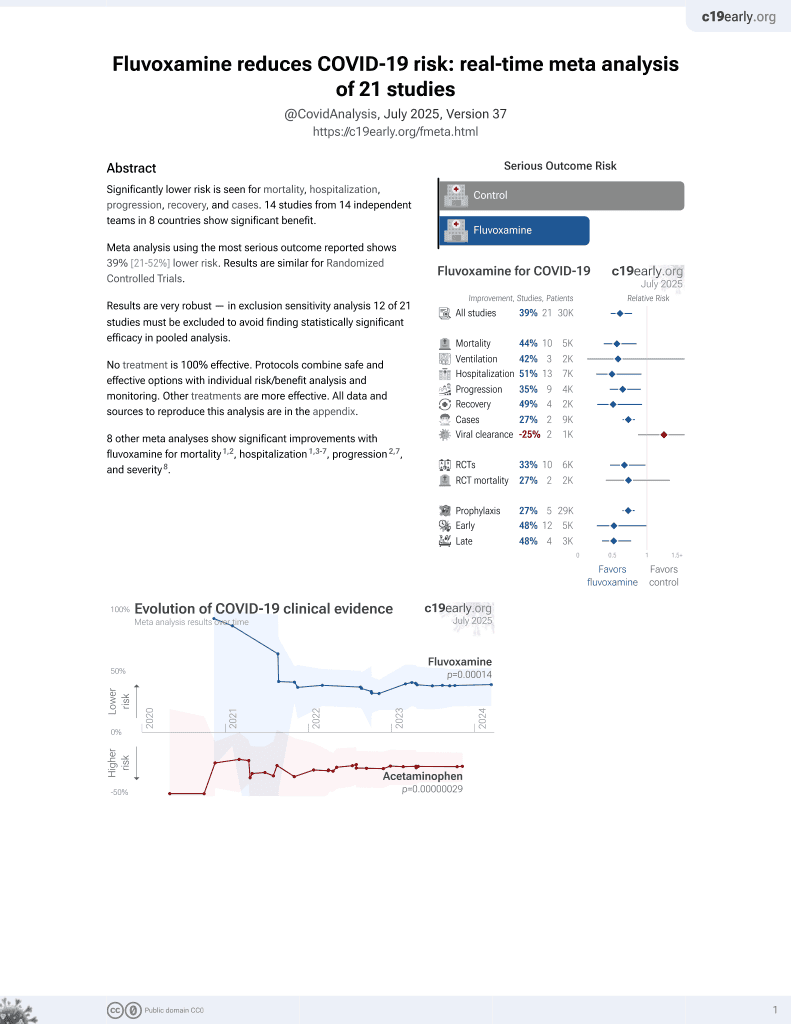
31st treatment shown to reduce risk in
November 2021, now with p = 0.00014 from 21 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro and in silico analysis supporting fluvoxamine for COVID-19. Authors investigated the mechanism of action of sigma-1 receptor (S1R) ligands for inhibiting SARS-CoV-2 replication, testing a series of S1R ligands and finding that some had potent antiviral activity against SARS-CoV-2 while others did not, even though they bound to S1R with similar affinity. Computational modeling suggested that specific ligand-protein interactions induce conformational changes in S1R that may be responsible for the antiviral effects. The findings provide insights into how S1R modulation could inhibit coronavirus replication and support further investigation of S1R-targeting drugs as potential COVID-19 treatments. However, a direct correlation between S1R affinity and antiviral potency was not found, indicating the mechanism may be more complex. Fluvoxamine is known to be a potent S1R agonist, with high binding affinity. This study found that some S1R ligands with nanomolar binding affinity had potent anti-SARS-CoV-2 effects in vitro. The computational modeling suggests the antiviral effects are due to conformational changes induced in S1R upon binding specific ligands. As a potent S1R agonist, fluvoxamine is likely to bind and induce conformational changes.
5 preclinical studies support the efficacy of fluvoxamine for COVID-19:
Fluvoxamine may inhibit SARS-CoV-2 cell entry by preventing the formation of ceramide platforms that facilitates viral uptake1, may help restore autophagic processes disrupted by NSP6, thereby reducing SARS-CoV-2 replication and improving host cellular defenses3, and may reduce COVID-19 thrombotic complications by inhibiting serotonin reuptake and decreasing platelet activation4.
1.
Alkafaas et al., Molecular docking as a tool for the discovery of novel insight about the role of acid sphingomyelinase inhibitors in SARS- CoV-2 infectivity, BMC Public Health, doi:10.1186/s12889-024-17747-z.
2.
Abatematteo et al., A conformational rearrangement of the SARS-CoV-2 host protein sigma-1 is required for antiviral activity: insights from a combined in-silico/in-vitro approach, Scientific Reports, doi:10.1038/s41598-023-39662-w.
Abatematteo et al., 7 Aug 2023, peer-reviewed, 13 authors.
Contact: giuseppe.mangiatordi@ic.cnr.it, carmen.abate@uniba.it.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
A conformational rearrangement of the SARS-CoV-2 host protein sigma-1 is required for antiviral activity: insights from a combined in-silico/in-vitro approach
Scientific Reports, doi:10.1038/s41598-023-39662-w
The development of effective drugs to treat coronavirus infections remains a significant challenge for the scientific community. Recent evidence reports on the sigma-1 receptor (S1R) as a key druggable host protein in the SARS-CoV-1 and SARS-CoV-2 interactomes and shows a potent antiviral activity against SARS-CoV-2 for the S1R antagonist PB28. To improve PB28 activity, we designed and tested a series of its analogues and identified a compound that is fourfold more potent against SARS-CoV-2 than PB28 itself. Interestingly, we found no direct correlation between S1R affinity and SARS-CoV-2 antiviral activity. Building on this, we employed comparative induced fit docking and molecular dynamics simulations to gain insights into the possible mechanism that occurs when specific ligandprotein interactions take place and that may be responsible for the observed antiviral activity. Our findings offer a possible explanation for the experimental observations, provide insights into the S1R conformational changes upon ligand binding and lay the foundation for the rational design of new S1R ligands with potent antiviral activity against SARS-CoV-2 and likely other viruses.
Abbreviations (S1R) Sigma-1 receptor (CNS) Central nervous system (SARS) Severe acute respiratory syndrome (MERS) Middle East respiratory syndrome (COVID-19) Coronavirus disease 2019 (FDA) Food and Drug Administration (S2R) Sigma-2 receptor (KO) Knocking out (KD) Knocking down (IFD) Induced Fit Docking
Author contributions All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by C.A. and G.F.M. Computational studies and analyses were performed by P.D., I.M., G.L. and G.F.M. Anti-SARS-CoV-2 assays were designed and performed by V.R., S.B. and M.V. Synthesis of S1R ligands was performed by F.S.A. The first draft of the manuscript was written by C.A. and G.F.M. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Competing interests The authors declare no competing interests.
References
Abate, 1-Cyclohexyl-4-(4-arylcyclohexyl)piperazines: Mixed σ and Human Δ8-Δ7 Sterol Isomerase Ligands with Antiproliferative and P-glycoprotein inhibitory activity, ChemMedChem
Abate, 2-Aminopyridine derivatives as potential σ2 receptor antagonists, ChemMedChem
Abate, 4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) with added polar functionality and reduced lipophilicity for potential use as positron emission tomography radiotracers, J. Med. Chem
Abate, Arylamides hybrids of two high-affinity σ2 receptor ligands as tools for the development of PET radiotracers, Eur J Med Chem
Abate, Development of 3,4-dihydroisoquinolin-1(2H)-one derivatives for the positron emission tomography (PET) imaging of σ2 receptors, Eur. J. Med. Chem
Abate, Mosier, Berardi, Glennon, A structure-affinity and comparative molecular field analysis of sigma-2 (sigma2) receptor ligands, Cent. Nerv. Syst. Agents Med. Chem
Abate, PB28, the Sigma-1 and Sigma-2 receptors modulator with potent anti-SARS-CoV-2 activity: a review about its pharmacological properties and structure affinity Relationships, Front. Pharmacol, doi:10.3389/fphar.2020.589810
Ali, Implication of in silico studies in the search for novel inhibitors against SARS-CoV-2, Arch. Pharm. (Weinheim)
Berardi, 4-(Tetralin-1-yl)-and 4-(Naphthalen-1-yl)alkyl derivatives of 1-cyclohexylpiperazine as σ receptor ligands with agonist σ2 activity, J. Med. Chem
Berardi, Exploring the importance of piperazine N-Atoms for σ2 receptor affinity and activity in a series of analogs of 1-cyclohexyl, J. Med. Chem
Berardi, Methyl substitution on the piperidine ring of N-[ω-(6-methoxynaphthalen-1-yl)alkyl] derivatives as a probe for selective binding and activity at the σ1 receptor, J. Med. Chem
Brimson, Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: The possible role of the sigma-1 receptor and autophagy, Expert Opin. Ther. Targets
Callaway, The coronavirus is mutating -Does it matter?, Nature
Canal-Rivero, Lower risk of SARS-CoV2 infection in individuals with severe mental disorders on antipsychotic treatment: A retrospective epidemiological study in a representative Spanish population, Schizophr. Res
Chakraborty, Sharma, Bhattacharya, Agoramoorthy, Lee, The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: lessons learned from major clinical studies, Front. Pharmacol, doi:10.3389/fphar.2021.704205
Commissioner, Coronavirus, COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults, FDA
Commissioner, Coronavirus, COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID
Commissioner, Coronavirus, COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant, FDA
Delre, Caporuscio, Saviano, Mangiatordi, Repurposing known drugs as covalent and non-covalent inhibitors of the SARS-CoV-2 papain-like protease, Front. Chem, doi:10.3389/fchem.2020.594009
Drugs, Fda, None
Feller, Zhang, Pastor, Brooks, Constant pressure molecular dynamics simulation: The Langevin piston method, J. Chem. Phys
Ferorelli, Design and evaluation of Naphthol-and Carbazole-containing fluorescent σ ligands as potential probes for receptor binding studies, J. Med. Chem
Gordon, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Gordon, Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms, Science
Gouda, Mégarbane, Molecular bases of serotonin reuptake inhibitor antidepressant-attributed effects in COVID-19: A new insight on the role of bradykinins, J. Personaliz. Med
Hanumegowda, Phospholipidosis as a function of basicity, lipophilicity, and volume of distribution of compounds, Chem. Res. Toxicol
Hashimoto, Suzuki, Hashimoto, Mechanisms of action of fluvoxamine for COVID-19: A historical review, Mol. Psychiatry
Johnston, Epik: pKa and Protonation State Prediction through Machine Learning, doi:10.26434/chemrxiv-2023-c6z8t
Kaminski, Friesner, Tirado-Rives, Jorgensen, Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides, J. Phys. Chem. B
Khani, Entezari-Maleki, Fluvoxamine and long COVID-19; a new role for sigma-1 receptor (S1R) agonists, Mol. Psychiatry
Lehrer, Rheinstein, Homozygosity for rs17775810 minor allele associated with reduced mortality of COVID-19 in the UK biobank cohort, In Vivo
Lu, OPLS4: improving force field accuracy on challenging regimes of chemical space, J. Chem. Theory Comput
Martyna, Tobias, Klein, Constant pressure molecular dynamics algorithms, J. Chem. Phys
Memish, Perlman, Van Kerkhove, Zumla, Middle East respiratory syndrome, Lancet
Meng, Xiao, Ji, Sun, Zhou, An open-like conformation of the sigma-1 receptor reveals its ligand entry pathway, Nat Commun
Niso, Sigma-2 receptor agonists as possible antitumor agents in resistant tumors: hints for collateral sensitivity, ChemMed-Chem
Onyango, Odhiambo, Angwenyi, Okoth, In silico identification of new anti-SARS-CoV-2 main protease (Mpro) molecules with pharmacokinetic properties from natural sources using molecular dynamics (MD) simulations and hierarchical virtual screening, J. Trop. Med
Perrone, N-aryl-or N-alkylpiperazine derivatives: The role of N-substituent on σ1, σ2, 5-HT1A and D2 receptor affinity, Med. Chem. Res
Research, Coronavirus, None
Sanjuán, Domingo-Calap, Mechanisms of viral mutation, Cell Mol Life Sci
Sars |, Factsheet, Cdc, None
Schmidt, Betz, Dror, Kruse, Structural basis for σ1 receptor ligand recognition, Nat Struct Mol Biol
Shaw Research, Schrödinger Release 2022-4: Desmond Molecular Dynamics System
Su, Su, Nakamura, Tsai, The sigma-1 receptor as a pluripotent modulator in living systems, Trends Pharmacol. Sci
Tsai, Pokrass, Klauer, De Credico, Su, Sigma-1 receptor chaperones in neurodegenerative and psychiatric disorders, Expert Opin Ther Targets
Tummino, Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2, Science
Vela, Repurposing Sigma-1 receptor ligands for COVID-19 therapy?, Front. Pharmacol, doi:10.3389/fphar.2020.582310
Yano, Pharmacological profiling of sigma 1 receptor ligands by novel receptor homomer assays, Neuropharmacology
Zhou, Wang, Tang, Nussinov, Cheng, Artificial intelligence in COVID-19 drug repurposing, Lancet Digital Health
DOI record:
{
"DOI": "10.1038/s41598-023-39662-w",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-023-39662-w",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The development of effective drugs to treat coronavirus infections remains a significant challenge for the scientific community. Recent evidence reports on the sigma-1 receptor (S1R) as a key druggable host protein in the SARS-CoV-1 and SARS-CoV-2 interactomes and shows a potent antiviral activity against SARS-CoV-2 for the S1R antagonist PB28. To improve PB28 activity, we designed and tested a series of its analogues and identified a compound that is fourfold more potent against SARS-CoV-2 than PB28 itself. Interestingly, we found no direct correlation between S1R affinity and SARS-CoV-2 antiviral activity. Building on this, we employed comparative induced fit docking and molecular dynamics simulations to gain insights into the possible mechanism that occurs when specific ligand–protein interactions take place and that may be responsible for the observed antiviral activity. Our findings offer a possible explanation for the experimental observations, provide insights into the S1R conformational changes upon ligand binding and lay the foundation for the rational design of new S1R ligands with potent antiviral activity against SARS-CoV-2 and likely other viruses.</jats:p>",
"alternative-id": [
"39662"
],
"article-number": "12798",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "24 May 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "7 August 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Abatematteo",
"given": "Francesca Serena",
"sequence": "first"
},
{
"affiliation": [],
"family": "Delre",
"given": "Pietro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mercurio",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rezelj",
"given": "Veronica V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siliqi",
"given": "Dritan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beaucourt",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lattanzi",
"given": "Gianluca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colabufo",
"given": "Nicola Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leopoldo",
"given": "Marcello",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saviano",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vignuzzi",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mangiatordi",
"given": "Giuseppe Felice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abate",
"given": "Carmen",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T12:02:15Z",
"timestamp": 1691409735000
},
"deposited": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T12:09:43Z",
"timestamp": 1691410183000
},
"funder": [
{
"DOI": "10.13039/501100004462",
"award": [
"PROGETTI DI RICERCA @CNR” (acronym DATIAMO)",
"PROGETTI DI RICERCA @CNR” (acronym DATIAMO)",
"PROGETTI DI RICERCA @CNR” (acronym DATIAMO)"
],
"doi-asserted-by": "publisher",
"name": "Consiglio Nazionale delle Ricerche"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
8
]
],
"date-time": "2023-08-08T04:25:08Z",
"timestamp": 1691468708118
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
8,
7
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T00:00:00Z",
"timestamp": 1691366400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
7
]
],
"date-time": "2023-08-07T00:00:00Z",
"timestamp": 1691366400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-023-39662-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-39662-w",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-39662-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
8,
7
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
7
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "39662_CR1",
"unstructured": "SARS | Basics Factsheet | CDC. https://www.cdc.gov/sars/about/fs-sars.html."
},
{
"DOI": "10.1016/S0140-6736(19)33221-0",
"author": "ZA Memish",
"doi-asserted-by": "publisher",
"first-page": "1063",
"journal-title": "Lancet",
"key": "39662_CR2",
"unstructured": "Memish, Z. A., Perlman, S., Van Kerkhove, M. D. & Zumla, A. Middle East respiratory syndrome. Lancet 395, 1063–1077 (2020).",
"volume": "395",
"year": "2020"
},
{
"key": "39662_CR3",
"unstructured": "Coronavirus Disease (COVID-19) Situation Reports. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports."
},
{
"DOI": "10.3389/fchem.2020.594009",
"author": "P Delre",
"doi-asserted-by": "publisher",
"journal-title": "Front. Chem.",
"key": "39662_CR4",
"unstructured": "Delre, P., Caporuscio, F., Saviano, M. & Mangiatordi, G. F. Repurposing known drugs as covalent and non-covalent inhibitors of the SARS-CoV-2 papain-like protease. Front. Chem. https://doi.org/10.3389/fchem.2020.594009 (2020).",
"year": "2020"
},
{
"DOI": "10.1002/ardp.202100360",
"author": "F Ali",
"doi-asserted-by": "publisher",
"first-page": "e2100360",
"journal-title": "Arch. Pharm. (Weinheim)",
"key": "39662_CR5",
"unstructured": "Ali, F. et al. Implication of in silico studies in the search for novel inhibitors against SARS-CoV-2. Arch. Pharm. (Weinheim) 355, e2100360 (2022).",
"volume": "355",
"year": "2022"
},
{
"DOI": "10.1016/S2589-7500(20)30192-8",
"author": "Y Zhou",
"doi-asserted-by": "publisher",
"first-page": "e667",
"journal-title": "Lancet Digital Health",
"key": "39662_CR6",
"unstructured": "Zhou, Y., Wang, F., Tang, J., Nussinov, R. & Cheng, F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digital Health 2, e667–e676 (2020).",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1155/2022/3697498",
"author": "H Onyango",
"doi-asserted-by": "publisher",
"first-page": "3697498",
"journal-title": "J. Trop. Med.",
"key": "39662_CR7",
"unstructured": "Onyango, H., Odhiambo, P., Angwenyi, D. & Okoth, P. In silico identification of new anti-SARS-CoV-2 main protease (Mpro) molecules with pharmacokinetic properties from natural sources using molecular dynamics (MD) simulations and hierarchical virtual screening. J. Trop. Med. 2022, 3697498 (2022).",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.704205",
"author": "C Chakraborty",
"doi-asserted-by": "publisher",
"journal-title": "Front. Pharmacol.",
"key": "39662_CR8",
"unstructured": "Chakraborty, C., Sharma, A. R., Bhattacharya, M., Agoramoorthy, G. & Lee, S.-S. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: lessons learned from major clinical studies. Front. Pharmacol. https://doi.org/10.3389/fphar.2021.704205 (2021).",
"year": "2021"
},
{
"key": "39662_CR9",
"unstructured": "Commissioner, O. of the. Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19. FDA https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 (2021)."
},
{
"key": "39662_CR10",
"unstructured": "Commissioner, O. of the. Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults. FDA https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain (2021)."
},
{
"key": "39662_CR11",
"unstructured": "Commissioner, O. of the. Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant. FDA https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron (2022)."
},
{
"DOI": "10.1007/s00018-016-2299-6",
"author": "R Sanjuán",
"doi-asserted-by": "publisher",
"first-page": "4433",
"journal-title": "Cell Mol Life Sci",
"key": "39662_CR12",
"unstructured": "Sanjuán, R. & Domingo-Calap, P. Mechanisms of viral mutation. Cell Mol Life Sci 73, 4433–4448 (2016).",
"volume": "73",
"year": "2016"
},
{
"DOI": "10.1038/d41586-020-02544-6",
"author": "E Callaway",
"doi-asserted-by": "publisher",
"first-page": "174",
"journal-title": "Nature",
"key": "39662_CR13",
"unstructured": "Callaway, E. The coronavirus is mutating — Does it matter?. Nature 585, 174–177 (2020).",
"volume": "585",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"author": "DE Gordon",
"doi-asserted-by": "publisher",
"first-page": "459",
"journal-title": "Nature",
"key": "39662_CR14",
"unstructured": "Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020).",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1126/science.abe9403",
"author": "DE Gordon",
"doi-asserted-by": "publisher",
"first-page": "eabe9403",
"journal-title": "Science",
"key": "39662_CR15",
"unstructured": "Gordon, D. E. et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science 370, eabe9403 (2020).",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.schres.2021.02.002",
"author": "M Canal-Rivero",
"doi-asserted-by": "publisher",
"first-page": "53",
"journal-title": "Schizophr. Res.",
"key": "39662_CR16",
"unstructured": "Canal-Rivero, M. et al. Lower risk of SARS-CoV2 infection in individuals with severe mental disorders on antipsychotic treatment: A retrospective epidemiological study in a representative Spanish population. Schizophr. Res. 229, 53–54 (2021).",
"volume": "229",
"year": "2021"
},
{
"DOI": "10.1038/s41380-021-01432-3",
"author": "Y Hashimoto",
"doi-asserted-by": "publisher",
"first-page": "1898",
"journal-title": "Mol. Psychiatry",
"key": "39662_CR17",
"unstructured": "Hashimoto, Y., Suzuki, T. & Hashimoto, K. Mechanisms of action of fluvoxamine for COVID-19: A historical review. Mol. Psychiatry 27, 1898–1907 (2022).",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41380-022-01545-3",
"author": "E Khani",
"doi-asserted-by": "publisher",
"first-page": "3562",
"journal-title": "Mol. Psychiatry",
"key": "39662_CR18",
"unstructured": "Khani, E. & Entezari-Maleki, T. Fluvoxamine and long COVID-19; a new role for sigma-1 receptor (S1R) agonists. Mol. Psychiatry 27, 3562–3562 (2022).",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.21873/invivo.12338",
"author": "S Lehrer",
"doi-asserted-by": "publisher",
"first-page": "965",
"journal-title": "In Vivo",
"key": "39662_CR19",
"unstructured": "Lehrer, S. & Rheinstein, P. H. Homozygosity for rs17775810 minor allele associated with reduced mortality of COVID-19 in the UK biobank cohort. In Vivo 35, 965–968 (2021).",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2020.582310",
"author": "JM Vela",
"doi-asserted-by": "publisher",
"journal-title": "Front. Pharmacol.",
"key": "39662_CR20",
"unstructured": "Vela, J. M. Repurposing Sigma-1 receptor ligands for COVID-19 therapy?. Front. Pharmacol. https://doi.org/10.3389/fphar.2020.582310 (2020).",
"year": "2020"
},
{
"key": "39662_CR21",
"unstructured": "Research, C. for D. E. and. Coronavirus (COVID-19) | Drugs. FDA https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs (2023)."
},
{
"DOI": "10.1038/s41594-018-0137-2",
"author": "HR Schmidt",
"doi-asserted-by": "publisher",
"first-page": "981",
"journal-title": "Nat Struct Mol Biol",
"key": "39662_CR22",
"unstructured": "Schmidt, H. R., Betz, R. M., Dror, R. O. & Kruse, A. C. Structural basis for σ1 receptor ligand recognition. Nat Struct Mol Biol 25, 981–987 (2018).",
"volume": "25",
"year": "2018"
},
{
"key": "39662_CR23",
"unstructured": "Schrödinger Release 2021–2: Protein Preparation Wizard; Epik, Schrödinger, LLC, (2021)."
},
{
"key": "39662_CR24",
"unstructured": "Schrödinger Release 2021–2: LigPrep, Schrödinger, LLC, (2021)."
},
{
"key": "39662_CR25",
"unstructured": "Schrödinger Release 2021–2: Glide, Schrödinger, LLC, New York, NY, (2021)."
},
{
"key": "39662_CR26",
"unstructured": "Schrödinger Release 2021–2: Induced Fit Docking protocol, Schrödinger, LLC, New York, NY, (2021)."
},
{
"DOI": "10.1021/jp003919d",
"author": "GA Kaminski",
"doi-asserted-by": "publisher",
"first-page": "6474",
"journal-title": "J. Phys. Chem. B",
"key": "39662_CR27",
"unstructured": "Kaminski, G. A., Friesner, R. A., Tirado-Rives, J. & Jorgensen, W. L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 105, 6474–6487 (2001).",
"volume": "105",
"year": "2001"
},
{
"key": "39662_CR28",
"unstructured": "Schrödinger Release 2022–4: Desmond Molecular Dynamics System, D. E. Shaw Research, New York, NY, 2021. Maestro-Desmond Interoperability Tools, Schrödinger, New York, NY, (2021)."
},
{
"DOI": "10.1021/acs.jctc.1c00302",
"author": "C Lu",
"doi-asserted-by": "publisher",
"first-page": "4291",
"journal-title": "J. Chem. Theory Comput.",
"key": "39662_CR29",
"unstructured": "Lu, C. et al. OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 17, 4291–4300 (2021).",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1063/1.470648",
"author": "SE Feller",
"doi-asserted-by": "publisher",
"first-page": "4613",
"journal-title": "J. Chem. Phys.",
"key": "39662_CR30",
"unstructured": "Feller, S. E., Zhang, Y., Pastor, R. W. & Brooks, B. R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 103, 4613–4621 (1995).",
"volume": "103",
"year": "1995"
},
{
"DOI": "10.1063/1.467468",
"author": "GJ Martyna",
"doi-asserted-by": "publisher",
"first-page": "4177",
"journal-title": "J. Chem. Phys.",
"key": "39662_CR31",
"unstructured": "Martyna, G. J., Tobias, D. J. & Klein, M. L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 101, 4177–4189 (1994).",
"volume": "101",
"year": "1994"
},
{
"key": "39662_CR32",
"unstructured": "Schrödinger Release 2022–4: QikProp, Schrödinger, LLC, New York, NY, (2021)."
},
{
"DOI": "10.26434/chemrxiv-2023-c6z8t",
"doi-asserted-by": "publisher",
"key": "39662_CR33",
"unstructured": "Johnston, R. C. et al. Epik: pKa and Protonation State Prediction through Machine Learning. Preprint at https://doi.org/10.26434/chemrxiv-2023-c6z8t (2023)."
},
{
"DOI": "10.3389/fphar.2020.589810",
"author": "C Abate",
"doi-asserted-by": "publisher",
"journal-title": "Front. Pharmacol.",
"key": "39662_CR34",
"unstructured": "Abate, C. et al. PB28, the Sigma-1 and Sigma-2 receptors modulator with potent anti–SARS-CoV-2 activity: a review about its pharmacological properties and structure affinity Relationships. Front. Pharmacol. https://doi.org/10.3389/fphar.2020.589810 (2020).",
"year": "2020"
},
{
"DOI": "10.1126/science.abi4708",
"author": "TA Tummino",
"doi-asserted-by": "publisher",
"first-page": "541",
"journal-title": "Science",
"key": "39662_CR35",
"unstructured": "Tummino, T. A. et al. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science 373, 541–547 (2021).",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1021/tx9003825",
"author": "UM Hanumegowda",
"doi-asserted-by": "publisher",
"first-page": "749",
"journal-title": "Chem. Res. Toxicol.",
"key": "39662_CR36",
"unstructured": "Hanumegowda, U. M. et al. Phospholipidosis as a function of basicity, lipophilicity, and volume of distribution of compounds. Chem. Res. Toxicol. 23, 749–755 (2010).",
"volume": "23",
"year": "2010"
},
{
"DOI": "10.1016/j.tips.2016.01.003",
"author": "T-P Su",
"doi-asserted-by": "publisher",
"first-page": "262",
"journal-title": "Trends Pharmacol. Sci.",
"key": "39662_CR37",
"unstructured": "Su, T.-P., Su, T.-C., Nakamura, Y. & Tsai, S.-Y. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol. Sci. 37, 262–278 (2016).",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1016/j.neuropharm.2018.01.042",
"author": "H Yano",
"doi-asserted-by": "publisher",
"first-page": "264",
"journal-title": "Neuropharmacology",
"key": "39662_CR38",
"unstructured": "Yano, H. et al. Pharmacological profiling of sigma 1 receptor ligands by novel receptor homomer assays. Neuropharmacology 133, 264–275 (2018).",
"volume": "133",
"year": "2018"
},
{
"DOI": "10.2174/1871524910909030246",
"author": "C Abate",
"doi-asserted-by": "publisher",
"first-page": "246",
"journal-title": "Cent. Nerv. Syst. Agents Med. Chem.",
"key": "39662_CR39",
"unstructured": "Abate, C., Mosier, P. D., Berardi, F. & Glennon, R. A. A structure-affinity and comparative molecular field analysis of sigma-2 (sigma2) receptor ligands. Cent. Nerv. Syst. Agents Med. Chem. 9, 246–257 (2009).",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1021/jm031026e",
"author": "F Berardi",
"doi-asserted-by": "publisher",
"first-page": "2308",
"journal-title": "J. Med. Chem.",
"key": "39662_CR40",
"unstructured": "Berardi, F. et al. 4-(Tetralin-1-yl)- and 4-(Naphthalen-1-yl)alkyl derivatives of 1-cyclohexylpiperazine as σ receptor ligands with agonist σ2 activity. J. Med. Chem. 47, 2308–2317 (2004).",
"volume": "47",
"year": "2004"
},
{
"DOI": "10.1021/jm070373b",
"author": "S Ferorelli",
"doi-asserted-by": "publisher",
"first-page": "4648",
"journal-title": "J. Med. Chem.",
"key": "39662_CR41",
"unstructured": "Ferorelli, S. et al. Design and evaluation of Naphthol- and Carbazole-containing fluorescent σ ligands as potential probes for receptor binding studies. J. Med. Chem. 50, 4648–4655 (2007).",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.1016/j.ejmech.2013.09.018",
"author": "C Abate",
"doi-asserted-by": "publisher",
"first-page": "920",
"journal-title": "Eur. J. Med. Chem.",
"key": "39662_CR42",
"unstructured": "Abate, C. et al. Development of 3,4-dihydroisoquinolin-1(2H)-one derivatives for the positron emission tomography (PET) imaging of σ2 receptors. Eur. J. Med. Chem. 69, 920–930 (2013).",
"volume": "69",
"year": "2013"
},
{
"author": "R Perrone",
"first-page": "201",
"journal-title": "Med. Chem. Res.",
"key": "39662_CR43",
"unstructured": "Perrone, R. et al. N-aryl- or N-alkylpiperazine derivatives: The role of N-substituent on σ1, σ2, 5-HT1A and D2 receptor affinity. Med. Chem. Res. 10, 201–207 (2000).",
"volume": "10",
"year": "2000"
},
{
"DOI": "10.1021/jm1013133",
"author": "C Abate",
"doi-asserted-by": "publisher",
"first-page": "1022",
"journal-title": "J. Med. Chem.",
"key": "39662_CR44",
"unstructured": "Abate, C. et al. Analogues of σ receptor ligand 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) with added polar functionality and reduced lipophilicity for potential use as positron emission tomography radiotracers. J. Med. Chem. 54, 1022–1032 (2011).",
"volume": "54",
"year": "2011"
},
{
"DOI": "10.1002/cmdc.201300291",
"author": "M Niso",
"doi-asserted-by": "publisher",
"first-page": "2026",
"journal-title": "ChemMedChem",
"key": "39662_CR45",
"unstructured": "Niso, M. et al. Sigma-2 receptor agonists as possible antitumor agents in resistant tumors: hints for collateral sensitivity. ChemMedChem 8, 2026–2035 (2013).",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1021/jm9007505",
"author": "F Berardi",
"doi-asserted-by": "publisher",
"first-page": "7817",
"journal-title": "J. Med. Chem.",
"key": "39662_CR46",
"unstructured": "Berardi, F. et al. Exploring the importance of piperazine N-Atoms for σ2 receptor affinity and activity in a series of analogs of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28). J. Med. Chem. 52, 7817–7828 (2009).",
"volume": "52",
"year": "2009"
},
{
"DOI": "10.1016/j.ejmech.2011.05.057",
"author": "C Abate",
"doi-asserted-by": "publisher",
"first-page": "4733",
"journal-title": "Eur J Med Chem",
"key": "39662_CR47",
"unstructured": "Abate, C. et al. Arylamides hybrids of two high-affinity σ2 receptor ligands as tools for the development of PET radiotracers. Eur J Med Chem 46, 4733–4741 (2011).",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.1002/cmdc.201200246",
"author": "C Abate",
"doi-asserted-by": "publisher",
"first-page": "1847",
"journal-title": "ChemMedChem",
"key": "39662_CR48",
"unstructured": "Abate, C. et al. 2-Aminopyridine derivatives as potential σ2 receptor antagonists. ChemMedChem 7, 1847–1857 (2012).",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1002/cmdc.201000371",
"author": "C Abate",
"doi-asserted-by": "publisher",
"first-page": "73",
"journal-title": "ChemMedChem",
"key": "39662_CR49",
"unstructured": "Abate, C. et al. 1-Cyclohexyl-4-(4-arylcyclohexyl)piperazines: Mixed σ and Human Δ8–Δ7 Sterol Isomerase Ligands with Antiproliferative and P-glycoprotein inhibitory activity. ChemMedChem 6, 73–80 (2011).",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1021/jm050654o",
"author": "F Berardi",
"doi-asserted-by": "publisher",
"first-page": "8237",
"journal-title": "J. Med. Chem.",
"key": "39662_CR50",
"unstructured": "Berardi, F. et al. Methyl substitution on the piperidine ring of N-[ω-(6-methoxynaphthalen-1-yl)alkyl] derivatives as a probe for selective binding and activity at the σ1 receptor. J. Med. Chem. 48, 8237–8244 (2005).",
"volume": "48",
"year": "2005"
},
{
"DOI": "10.1038/s41467-022-28946-w",
"author": "F Meng",
"doi-asserted-by": "publisher",
"first-page": "1267",
"journal-title": "Nat Commun",
"key": "39662_CR51",
"unstructured": "Meng, F., Xiao, Y., Ji, Y., Sun, Z. & Zhou, X. An open-like conformation of the sigma-1 receptor reveals its ligand entry pathway. Nat Commun 13, 1267 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/jpm12091487",
"author": "AS Gouda",
"doi-asserted-by": "publisher",
"first-page": "1487",
"journal-title": "J. Personaliz. Med.",
"key": "39662_CR52",
"unstructured": "Gouda, A. S. & Mégarbane, B. Molecular bases of serotonin reuptake inhibitor antidepressant-attributed effects in COVID-19: A new insight on the role of bradykinins. J. Personaliz. Med. 12, 1487 (2022).",
"volume": "12",
"year": "2022"
},
{
"author": "S-YA Tsai",
"first-page": "1461",
"journal-title": "Expert Opin Ther Targets",
"key": "39662_CR53",
"unstructured": "Tsai, S.-Y.A., Pokrass, M. J., Klauer, N. R., De Credico, N. E. & Su, T.-P. Sigma-1 receptor chaperones in neurodegenerative and psychiatric disorders. Expert Opin Ther Targets 18, 1461–1476 (2014).",
"volume": "18",
"year": "2014"
},
{
"DOI": "10.1080/14728222.2021.1952987",
"author": "JM Brimson",
"doi-asserted-by": "publisher",
"first-page": "435",
"journal-title": "Expert Opin. Ther. Targets",
"key": "39662_CR54",
"unstructured": "Brimson, J. M. et al. Drugs that offer the potential to reduce hospitalization and mortality from SARS-CoV-2 infection: The possible role of the sigma-1 receptor and autophagy. Expert Opin. Ther. Targets 25, 435–449 (2021).",
"volume": "25",
"year": "2021"
}
],
"reference-count": 54,
"references-count": 54,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-023-39662-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "A conformational rearrangement of the SARS-CoV-2 host protein sigma-1 is required for antiviral activity: insights from a combined in-silico/in-vitro approach",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}