Nebulized inhalation of plasma-activated water in the treatment of progressive moderate COVID-19 patients with antiviral treatment failure: a randomized controlled pilot trial
et al., BMC Infectious Diseases, doi:10.1186/s12879-024-09886-w, ChiCTR2300078706, Sep 2024
RCT 23 moderate COVID-19 patients with antiviral treatment failure, showing nebulized plasma-activated water (PAW) improved chest CT and accelerated cough resolution compared to saline control, however there was no significant difference in progression. No adverse reactions were reported. Authors suggest that PAW may disrupt SARS-CoV-2 binding and replication and have anti-inflammatory effects in the lungs.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
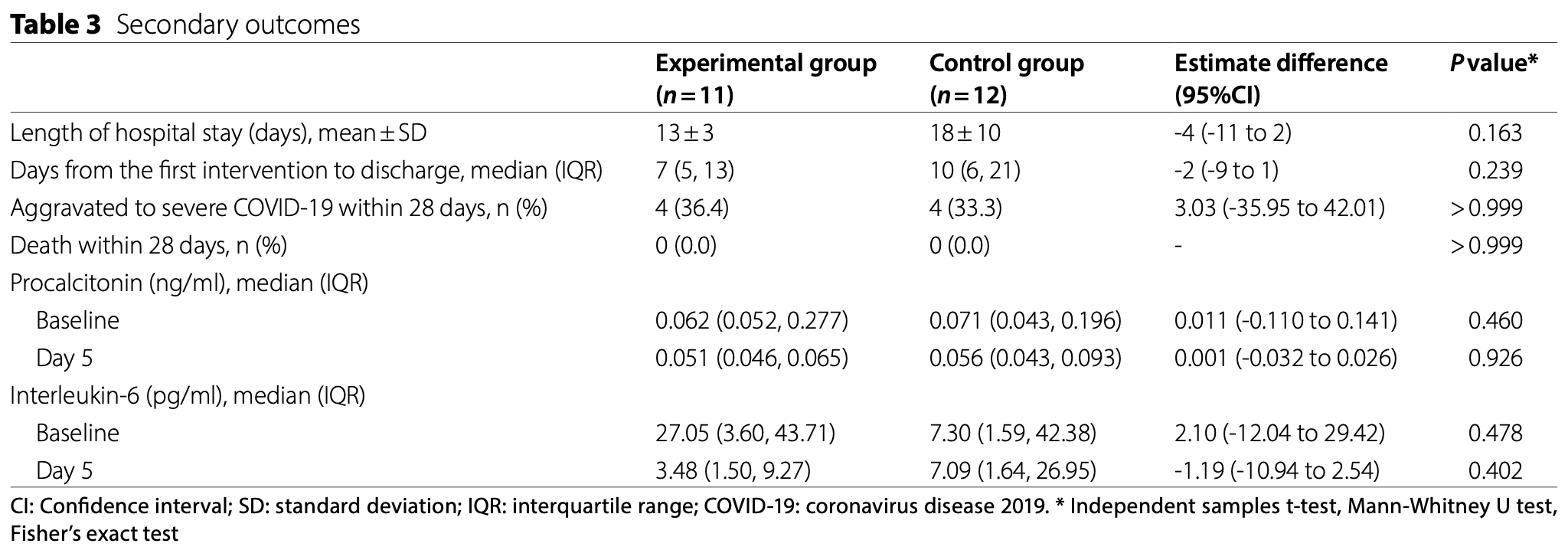
risk of progression, 9.1% higher, RR 1.09, p = 1.00, treatment 4 of 11 (36.4%), control 4 of 12 (33.3%).
|
|
no CT improvement, 72.7% lower, RR 0.27, p = 0.04, treatment 2 of 11 (18.2%), control 8 of 12 (66.7%), NNT 2.1.
|
|
hospitalization time, 27.8% lower, relative time 0.72, p = 0.16, treatment 11, control 12.
|
|
time to discharge, 30.0% lower, relative time 0.70, p = 0.24, treatment 11, control 12.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhao et al., 12 Sep 2024, Single Blind Randomized Controlled Trial, China, peer-reviewed, 12 authors, study period January 2023 - July 2023, trial ChiCTR2300078706.
Contact: liudingxin@mail.xjtu.edu.cn, songlq@fmmu.edu.cn.
Nebulized inhalation of plasma-activated water in the treatment of progressive moderate COVID-19 patients with antiviral treatment failure: a randomized controlled pilot trial
BMC Infectious Diseases, doi:10.1186/s12879-024-09886-w
Background Antiviral drugs show significant efficacy in non-severe COVID-19 cases, yet there remains a subset of moderate COVID-19 patients whose pneumonia continues to progress post a complete course of treatment. Plasma-activated water (PAW) possesses anti-SARS-CoV-2 properties. To explore the potential of PAW in improving pneumonia in COVID-19 patients following antiviral treatment failure, we conducted this study. Methods This was a randomized, controlled trial. Moderate COVID-19 patients with antiviral treatment failure were randomly assigned to the experimental group or the control group. They inhaled nebulized PAW or saline respectively. This was done twice daily for four consecutive days. We assessed improvement in chest CT on day 5, the rate of symptom resolution within 10 days, and safety. Results A total of 23 participants were included, with 11 receiving PAW and 12 receiving saline. The baseline characteristics of both groups were comparable. The experimental group showed a higher improvement rate in chest CT on day 5 (81.8% vs. 33.3%, p = 0.036). The cumulative disappearance rate of cough within 10 days was higher in the experimental group. Within 28 days, 4 patients in each group progressed to severe illness, and no patients died. No adverse reactions were reported from inhaling nebulized PAW.
Declarations Ethics approval and consent to participate The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of the First Affiliated Hospital of the Air Force Medical University (Approval No.: KY20232004-C-1, January 5, 2023), registered with the Chinese Clinical Trial Registry (Registration No.: ChiCTR2300078706). Informed consent was obtained from all subjects involved in the study.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Amini, Hosseini, Fatollah, Mirpour, Ghoranneviss et al., Beneficial effects of cold atmospheric plasma on inflammatory phase of diabetic foot ulcers; a randomized clinical trial, J Diabetes Metabolic Disorders
Anderson, Atkins, Bäckman, Cipolla, Clark et al., Inhaled medicines: past, present, and future, Pharmacol Rev
Azizmohammadi, Azizmohammadi, Dahmardeh, Azargashb, Shokouh et al., Analysis of 239 ordinary and severe cases of COVID-19: clinical features and treatment, Eur J Transl Myol
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, New Engl J Med
Bisag, Isabelli, Laghi, Laurita, Dirani et al., Cold atmospheric plasma decontamination of SARS-CoV-2 bioaerosols, Plasma Process Polym
Bisag, Isabelli, Laurita, Bucci, Capelli et al., Cold atmospheric plasma inactivation of aerosolized microdroplets containing bacteria and purified SARS-CoV-2 RNA to contrast airborne indoor transmission, Plasma Process Polym
Braman, Postinfectious cough, Chest
Choi, Song, Lee, Hong, Kim, Inhibition of inflammatory reactions in 2,4-dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by nonthermal plasma, Sci Rep-UK
Cortázar, Megía-Macías, Moreno, Brun, Gómez-Casado, Vulnerability of SARS-CoV-2 and PR8 H1N1 virus to cold atmospheric plasma activated media, Sci Rep-UK
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of Coronavirus disease 2019 in China, New Engl J Med
Guo, Lv, Wu, Wang, Wu et al., Safety and efficacy of plasma-activated water on prolonged viral shedding of COVID-19 patients: a randomized controlled trial, Plasma Process Polym
Guo, Xu, Gou, Liu, Zhao et al., Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasmaactivated water, Appl Environ Microb
Guo, Yao, Yang, Zhang, Qi et al., Plasma-activated water: an alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection, Chem Eng J
Gül, Gonen, Jones, Taşlı, Zararsız et al., A pilot study for treatment of severe COVID-19 pneumonia by aerosolized formulation of convalescent human immune plasma exosomes (ChipEXO™), Front Immunol
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, New Engl J Med
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hwang, Jeon, Wang, Kim, Kim et al., Design and medical effects of a vaginal cleaning device generating plasmaactivated water with antimicrobial activity on bacterial vaginosis, Plasma
Jang, Bok, Ahn, Kim, Kang, Human trial for the effect of plasmaactivated water spray on vaginal cleaning in patients with bacterial vaginosis, Med Sci
Kabinger, Stiller, Schmitzová, Dienemann, Kokic et al., Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol
Kaushik, Ghimire, Li, Adhikari, Veerana et al., Biological and medical applications of plasma-activated media, water and solutions, Biol Chem
Khanikar, Kalita, Kalita, Kashyap, Das et al., Cold atmospheric pressure plasma for attenuation of SARS-CoV-2 spike protein binding to ACE2 protein and the RNA deactivation, Rsc Adv
Kim, Kim, Applications of plasma-activated liquid in the medical field, Biomedicines
Lee, Lee, Kim, Han, Kang et al., Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice, Sci Rep-UK
Lee, Lee, Kim, Won, Kim, Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression, Sci Rep-UK
Li, Liu, Liu, Hu, Yang et al., Analysis of risk factors for 24 patients with COVID-19 developing from moderate to severe condition, Front Cell Infect Mi
Li, Zhan, Yang, Tu, Hu et al., Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific singledomain antibody, Cell
Liao, Liu, Yuan, Wen, Xu et al., Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19, Nat Med
Loizou, Kniazeva, Apostolou, Kornev, Kostevitch et al., Effect of cold atmospheric plasma on SARS-CoV-2 inactivation: a pilot study in the hospital environment, COVID
Luo, Yu, Gou, Li, Sun et al., Histopathologic findings in the explant lungs of a patient with COVID-19 treated with bilateral orthotopic lung transplant, Transplantation
Nastasa, Pasca, Malancus, Bostanaru, Ailincai et al., Toxicity assessment of long-term exposure to nonthermal plasma activated water in mice, Int J Mol Sci
Ng, Correia, Seagal, Degoey, Schrimpf et al., Antiviral drug discovery for the treatment of COVID-19 infections, Viruses-Basel
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19, Science
Poulakou, Barakat, Israel, Bacci, Ribavirin aerosol in hospitalized adults with respiratory distress and COVID-19: an open-label trial, Clin Transl Sci
Santi Laurini, Montanaro, Motola, Safety profile of Molnupiravir in the treatment of COVID-19: a descriptive study based on FAERS data, J Clin Med
Seebauer, Freund, Hasse, Miller, Segebarth et al., Effects of cold physical plasma on oral lichen planus: an in vitro study (effects of CAP on OLP), Oral Dis
Shen, Yu, Cai, Zhou, Sheng et al., Quantitative computed tomography analysis for stratifying the severity of Coronavirus disease 2019, J Pharm Anal
Song, Hui, Hull, Birring, Mcgarvey et al., Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses, Lancet Respiratory Med
Tamasauskiene, Sitkauskiene, Immune system in the pathogenesis of chronic cough, Immunol Lett
Wai, Chan, Cheung, Wang, Chan et al., Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health -Western Pac
Wang, Zhou, Zhou, Li, Weerasinghe et al., Cold atmospheric plasma for preventing infection of viruses that use ACE2 for entry, Theranostics
Xu D, Cui, Xu, Tian, Liu et al., Systemic study on the safety of immuno-deficient nude mice treated by atmospheric plasma-activated water, Plasma Sci Technol
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respiratory Med
Zhai, Xu, Li, Guo, Chen et al., Successful treatment of vitiligo with cold atmospheric plasma-activated hydrogel, J Invest Dermatol
Zhang, Chua, Selva, Kedzierski, Ashhurst et al., Immune responses in COVID-19 respiratory tract and blood reveal mechanisms of disease severity, Res Sq
Zheng, Zhou, Zhou, Ye, Huang et al., SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial, Int J Infect Dis
Zhou, Guo, Li, Fang, Chen et al., Coronavirus disease 2019: initial chest CT findings, Eur Radiol
Zhou, Zhou, Wang, Xian, Mai-Prochnow et al., Plasma-activated water: generation, origin of reactive species and biological applications, J Phys D
Zhuang, Xu, Wu, Yang, Lin et al., Postmarketing safety concerns with nirmatrelvir: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system, Brit J Clin Pharmaco
DOI record:
{
"DOI": "10.1186/s12879-024-09886-w",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-024-09886-w",
"alternative-id": [
"9886"
],
"article-number": "960",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "12 April 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 September 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "12 September 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical Ethics Committee of the First Affiliated Hospital of the Air Force Medical University (Approval No.: KY20232004-C-1, January 5, 2023), registered with the Chinese Clinical Trial Registry (Registration No.: ChiCTR2300078706). Informed consent was obtained from all subjects involved in the study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Zhao",
"given": "Heng",
"sequence": "first"
},
{
"affiliation": [],
"family": "Meng",
"given": "Wanting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lv",
"given": "Xing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cai",
"given": "Zhigui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Xingxing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Zifeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rong",
"given": "Mingzhe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shen",
"given": "Cong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Dingxin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Liqiang",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T09:05:01Z",
"timestamp": 1726131901000
},
"deposited": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T09:05:33Z",
"timestamp": 1726131933000
},
"funder": [
{
"award": [
"No. 2022JZ-58"
],
"name": "The Key projects of basic research of natural science in Shaanxi Province"
},
{
"award": [
"No. XJZT21CZ06"
],
"name": "The Discipline Promotion Plan of Xijing Hospital"
},
{
"award": [
"No. 2021JSTS36"
],
"name": "The Clinical Research Program of Air Force Military Medical University"
},
{
"award": [
"No. 2021A006"
],
"name": "The Shaanxi Provincial Health Scientific Research Fund Project"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
13
]
],
"date-time": "2024-09-13T00:48:09Z",
"timestamp": 1726188489039
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
9,
12
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T00:00:00Z",
"timestamp": 1726099200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T00:00:00Z",
"timestamp": 1726099200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09886-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-024-09886-w/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-024-09886-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
9,
12
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3390/v14050961",
"author": "TI Ng",
"doi-asserted-by": "publisher",
"first-page": "961",
"issue": "5",
"journal-title": "VIRUSES-BASEL",
"key": "9886_CR1",
"unstructured": "Ng TI, Correia I, Seagal J, DeGoey DA, Schrimpf MR, Hardee DJ, Noey EL, Kati WM. Antiviral drug discovery for the treatment of COVID-19 infections. Viruses-Basel. 2022;14(5):961.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "crossref",
"key": "9886_CR2",
"unstructured": "Owen DR, Allerton CMN, Anderson AS, Aschenbrenner L, Avery M, Berritt S, Boras B, Cardin RD, Carlo A, Coffman KJ et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593."
},
{
"DOI": "10.1038/s41594-021-00651-0",
"author": "F Kabinger",
"doi-asserted-by": "publisher",
"first-page": "740",
"issue": "9",
"journal-title": "NAT STRUCT MOL BIOL",
"key": "9886_CR3",
"unstructured": "Kabinger F, Stiller C, Schmitzová J, Dienemann C, Kokic G, Hillen HS, Höbartner C, Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740–6.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "NEW ENGL J MED",
"key": "9886_CR4",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, Baniecki M, Hendrick VM, Damle B, Simón-Campos A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. New Engl J Med. 2022;386(15):1397–408.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "NEW ENGL J MED",
"key": "9886_CR5",
"unstructured": "Jayk Bernal A, Gomes Da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. New Engl J Med. 2022;386(6):509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"author": "AK Wai",
"doi-asserted-by": "publisher",
"first-page": "100602",
"journal-title": "Lancet Reg Health - Western Pac",
"key": "9886_CR6",
"unstructured": "Wai AK, Chan CY, Cheung AW, Wang K, Chan SC, Lee TT, Luk LY, Yip ET, Ho JW, Tsui OW, et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health - Western Pac. 2023;30:100602.",
"volume": "30",
"year": "2023"
},
{
"DOI": "10.1111/bcp.15783",
"author": "W Zhuang",
"doi-asserted-by": "publisher",
"first-page": "2830",
"issue": "9",
"journal-title": "BRIT J CLIN PHARMACO",
"key": "9886_CR7",
"unstructured": "Zhuang W, Xu J, Wu Y, Yang J, Lin X, Liao Y, Wan J, Weng L, Lin W. Post-marketing safety concerns with nirmatrelvir: a disproportionality analysis of spontaneous reports submitted to the FDA adverse event reporting system. Brit J Clin Pharmaco. 2023;89(9):2830–42.",
"volume": "89",
"year": "2023"
},
{
"DOI": "10.3390/jcm12010034",
"author": "G Santi Laurini",
"doi-asserted-by": "publisher",
"first-page": "34",
"issue": "1",
"journal-title": "J CLIN MED",
"key": "9886_CR8",
"unstructured": "Santi Laurini G, Montanaro N, Motola D. Safety profile of Molnupiravir in the treatment of COVID-19: a descriptive study based on FAERS data. J Clin Med. 2023;12(1):34.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1515/hsz-2018-0226",
"author": "NK Kaushik",
"doi-asserted-by": "publisher",
"first-page": "39",
"issue": "1",
"journal-title": "BIOL CHEM",
"key": "9886_CR9",
"unstructured": "Kaushik NK, Ghimire B, Li Y, Adhikari M, Veerana M, Kaushik N, Jha N, Adhikari B, Lee S, Masur K, et al. Biological and medical applications of plasma-activated media, water and solutions. Biol Chem. 2019;400(1):39.",
"volume": "400",
"year": "2019"
},
{
"DOI": "10.3390/biomedicines9111700",
"doi-asserted-by": "crossref",
"key": "9886_CR10",
"unstructured": "Kim S, Kim C. Applications of plasma-activated liquid in the medical field. Biomedicines. 2021;9(11):1700."
},
{
"DOI": "10.1088/1361-6463/ab81cf",
"author": "R Zhou",
"doi-asserted-by": "publisher",
"first-page": "303001",
"issue": "30",
"journal-title": "J Phys D",
"key": "9886_CR11",
"unstructured": "Zhou R, Zhou R, Wang P, Xian Y, Mai-Prochnow A, Lu X, Cullen PJ, Ostrikov KK, Bazaka K. Plasma-activated water: generation, origin of reactive species and biological applications. J Phys D. 2020;53(30):303001.",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1002/ppap.202100133",
"doi-asserted-by": "crossref",
"key": "9886_CR12",
"unstructured": "Bisag A, Isabelli P, Laghi G, Laurita R, Dirani G, Taddei F, Bucci C, Capelli F, Gherardi M, Paglianti A et al. Cold atmospheric plasma decontamination of SARS-CoV‐2 bioaerosols. Plasma Process Polym. 2022;19(3)."
},
{
"DOI": "10.3390/covid2100100",
"author": "C Loizou",
"doi-asserted-by": "publisher",
"first-page": "1396",
"issue": "10",
"journal-title": "COVID",
"key": "9886_CR13",
"unstructured": "Loizou C, Kniazeva V, Apostolou T, Kornev A, Kostevitch S, Roslyakov E, Constantinou C, Hadjihannas L. Effect of cold atmospheric plasma on SARS-CoV-2 inactivation: a pilot study in the hospital environment. COVID. 2022;2(10):1396–404.",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1002/ppap.202000154",
"doi-asserted-by": "crossref",
"key": "9886_CR14",
"unstructured": "Bisag A, Isabelli P, Laurita R, Bucci C, Capelli F, Dirani G, Gherardi M, Laghi G, Paglianti A, Sambri V et al. Cold atmospheric plasma inactivation of aerosolized microdroplets containing bacteria and purified SARS-CoV‐2 RNA to contrast airborne indoor transmission. Plasma Process Polym. 2020;17(10)."
},
{
"DOI": "10.1016/j.cej.2020.127742",
"author": "L Guo",
"doi-asserted-by": "publisher",
"first-page": "127742",
"journal-title": "CHEM ENG J",
"key": "9886_CR15",
"unstructured": "Guo L, Yao Z, Yang L, Zhang H, Qi Y, Gou L, Xi W, Liu D, Zhang L, Cheng Y, et al. Plasma-activated water: an alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem Eng J. 2021;421:127742.",
"volume": "421",
"year": "2021"
},
{
"DOI": "10.1088/2058-6272/aa9842",
"author": "D XU",
"doi-asserted-by": "publisher",
"first-page": "44003",
"issue": "4",
"journal-title": "Plasma Sci Technol",
"key": "9886_CR16",
"unstructured": "XU D, CUI Q, XU Y, WANG B, TIAN M, LI Q, LIU Z, LIU D, CHEN H, KONG MG. Systemic study on the safety of immuno-deficient nude mice treated by atmospheric plasma-activated water. Plasma Sci Technol. 2018;20(4):44003.",
"volume": "20",
"year": "2018"
},
{
"DOI": "10.3390/ijms222111534",
"author": "V Nastasa",
"doi-asserted-by": "publisher",
"first-page": "11534",
"issue": "21",
"journal-title": "Int J Mol Sci",
"key": "9886_CR17",
"unstructured": "Nastasa V, Pasca A, Malancus R, Bostanaru A, Ailincai L, Ursu E, Vasiliu A, Minea B, Hnatiuc E, Mares M. Toxicity assessment of long-term exposure to non-thermal plasma activated water in mice. Int J Mol Sci. 2021;22(21):11534.",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.3390/plasma3040016",
"author": "Y Hwang",
"doi-asserted-by": "publisher",
"first-page": "204",
"issue": "4",
"journal-title": "Plasma",
"key": "9886_CR18",
"unstructured": "Hwang Y, Jeon H, Wang GY, Kim HK, Kim J, Ahn DK, Choi JS, Jang Y. Design and medical effects of a vaginal cleaning device generating plasma-activated water with antimicrobial activity on bacterial vaginosis. Plasma. 2020;3(4):204–13.",
"volume": "3",
"year": "2020"
},
{
"author": "Y Jang",
"first-page": "33",
"issue": "2",
"journal-title": "Med Sci",
"key": "9886_CR19",
"unstructured": "Jang Y, Bok J, Ahn DK, Kim C, Kang J. Human trial for the effect of plasma-activated water spray on vaginal cleaning in patients with bacterial vaginosis. Med Sci. 2022;10(2):33.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1002/ppap.202300195",
"doi-asserted-by": "crossref",
"key": "9886_CR20",
"unstructured": "Guo X, Lv X, Wu Y, Wang M, Wu S, Guo L, Wang Z, Shi Z, Huang R, Zhang H et al. Safety and efficacy of plasma-activated water on prolonged viral shedding of COVID‐19 patients: a randomized controlled trial. Plasma Process Polym. 2023."
},
{
"DOI": "10.1124/pharmrev.120.000108",
"author": "S Anderson",
"doi-asserted-by": "publisher",
"first-page": "48",
"issue": "1",
"journal-title": "PHARMACOL REV",
"key": "9886_CR21",
"unstructured": "Anderson S, Atkins P, Bäckman P, Cipolla D, Clark A, Daviskas E, Disse B, Entcheva-Dimitrov P, Fuller R, Gonda I, et al. Inhaled medicines: past, present, and future. Pharmacol Rev. 2022;74(1):48–118.",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.963309",
"doi-asserted-by": "crossref",
"key": "9886_CR22",
"unstructured": "Gül F, Gonen ZB, Jones OY, Taşlı NP, Zararsız G, Ünal E, Özdarendeli A, Şahin F, Eken A, Yılmaz S et al. A pilot study for treatment of severe COVID-19 pneumonia by aerosolized formulation of convalescent human immune plasma exosomes (ChipEXO™). Front Immunol. 2022;13."
},
{
"DOI": "10.1016/j.cell.2022.03.009",
"author": "C Li",
"doi-asserted-by": "publisher",
"first-page": "1389",
"issue": "8",
"journal-title": "Cell",
"key": "9886_CR23",
"unstructured": "Li C, Zhan W, Yang Z, Tu C, Hu G, Zhang X, Song W, Du S, Zhu Y, Huang K, et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell. 2022;185(8):1389–401.",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1111/cts.13436",
"author": "G Poulakou",
"doi-asserted-by": "publisher",
"first-page": "165",
"issue": "1",
"journal-title": "Clin Transl Sci",
"key": "9886_CR24",
"unstructured": "Poulakou G, Barakat M, Israel RJ, Bacci MR. Ribavirin aerosol in hospitalized adults with respiratory distress and COVID-19: an open‐label trial. Clin Transl Sci. 2023;16(1):165–74.",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2020.07.053",
"author": "F Zheng",
"doi-asserted-by": "publisher",
"first-page": "84",
"journal-title": "INT J INFECT DIS",
"key": "9886_CR25",
"unstructured": "Zheng F, Zhou Y, Zhou Z, Ye F, Huang B, Huang Y, Ma J, Zuo Q, Tan X, Xie J, et al. SARS-CoV-2 clearance in COVID-19 patients with Novaferon treatment: a randomized, open-label, parallel-group trial. Int J Infect Dis. 2020;99:84–91.",
"volume": "99",
"year": "2020"
},
{
"DOI": "10.1038/s41598-021-04360-y",
"doi-asserted-by": "crossref",
"key": "9886_CR26",
"unstructured": "Cortázar OD, Megía-Macías A, Moreno S, Brun A, Gómez-Casado E. Vulnerability of SARS-CoV-2 and PR8 H1N1 virus to cold atmospheric plasma activated media. Sci Rep-UK. 2022;12(1)."
},
{
"DOI": "10.1016/j.jpha.2020.03.004",
"author": "C Shen",
"doi-asserted-by": "publisher",
"first-page": "123",
"issue": "2",
"journal-title": "J PHARM ANAL",
"key": "9886_CR27",
"unstructured": "Shen C, Yu N, Cai S, Zhou J, Sheng J, Liu K, Zhou H, Guo Y, Niu G. Quantitative computed tomography analysis for stratifying the severity of Coronavirus disease 2019. J Pharm Anal. 2020;10(2):123–9.",
"volume": "10",
"year": "2020"
},
{
"key": "9886_CR28",
"unstructured": "Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. https://www.fda.gov/media/142143/download. Accessed 13 August 2024."
},
{
"DOI": "10.4081/ejtm.2021.9579",
"doi-asserted-by": "crossref",
"key": "9886_CR29",
"unstructured": "Azizmohammadi S, Azizmohammadi S, Dahmardeh S, Azargashb H, Shokouh SJH, Mohajeri-Iravani M, Mobasher M, Soleiman-Meigooni S, Zabihi M. Analysis of 239 ordinary and severe cases of COVID-19: clinical features and treatment. Eur J Transl Myol. 2021;31(3)."
},
{
"DOI": "10.3389/fcimb.2020.548582",
"doi-asserted-by": "crossref",
"key": "9886_CR30",
"unstructured": "Li D, Liu C, Liu J, Hu J, Yang Y, Zhou Y. Analysis of risk factors for 24 patients with COVID-19 developing from moderate to severe condition. Front Cell Infect Mi. 2020;10."
},
{
"DOI": "10.1007/s00330-020-06816-7",
"author": "Z Zhou",
"doi-asserted-by": "publisher",
"first-page": "4398",
"issue": "8",
"journal-title": "EUR RADIOL",
"key": "9886_CR31",
"unstructured": "Zhou Z, Guo D, Li C, Fang Z, Chen L, Yang R, Li X, Zeng W. Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 2020;30(8):4398–406.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1128/AEM.00726-18",
"doi-asserted-by": "crossref",
"key": "9886_CR32",
"unstructured": "Guo L, Xu R, Gou L, Liu Z, Zhao Y, Liu D, Zhang L, Chen H, Kong MG. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl Environ Microb. 2018;84(17)."
},
{
"DOI": "10.7150/thno.70098",
"author": "P Wang",
"doi-asserted-by": "publisher",
"first-page": "2811",
"issue": "6",
"journal-title": "THERANOSTICS",
"key": "9886_CR33",
"unstructured": "Wang P, Zhou R, Zhou R, Li W, Weerasinghe J, Chen S, Rehm BHA, Zhao L, Frentiu FD, Zhang Z, et al. Cold atmospheric plasma for preventing infection of viruses that use ACE2 for entry. Theranostics. 2022;12(6):2811–32.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1039/D2RA00009A",
"author": "RR Khanikar",
"doi-asserted-by": "publisher",
"first-page": "9466",
"issue": "15",
"journal-title": "RSC ADV",
"key": "9886_CR34",
"unstructured": "Khanikar RR, Kalita M, Kalita P, Kashyap B, Das S, Khan MR, Bailung H, Sankaranarayanan K. Cold atmospheric pressure plasma for attenuation of SARS-CoV-2 spike protein binding to ACE2 protein and the RNA deactivation. Rsc Adv. 2022;12(15):9466–72.",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1097/TP.0000000000003412",
"author": "W Luo",
"doi-asserted-by": "publisher",
"first-page": "e329",
"issue": "11",
"journal-title": "Transplantation",
"key": "9886_CR35",
"unstructured": "Luo W, Yu H, Gou J, Li X, Sun Y, Li J, He J, Liu L. Histopathologic findings in the explant lungs of a patient with COVID-19 treated with bilateral orthotopic lung transplant. Transplantation. 2020;104(11):e329–31.",
"volume": "104",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"author": "Z Xu",
"doi-asserted-by": "publisher",
"first-page": "420",
"issue": "4",
"journal-title": "Lancet Respiratory Med",
"key": "9886_CR36",
"unstructured": "Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respiratory Med. 2020;8(4):420–2.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0901-9",
"author": "M Liao",
"doi-asserted-by": "publisher",
"first-page": "842",
"issue": "6",
"journal-title": "NAT MED",
"key": "9886_CR37",
"unstructured": "Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Cheng L, Li J, Wang X, Wang F, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–4.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s40200-020-00577-2",
"author": "MR Amini",
"doi-asserted-by": "publisher",
"first-page": "895",
"issue": "2",
"journal-title": "J Diabetes Metabolic Disorders",
"key": "9886_CR38",
"unstructured": "Amini MR, Sheikh Hosseini M, Fatollah S, Mirpour S, Ghoranneviss M, Larijani B, Mohajeri-Tehrani MR, Khorramizadeh MR. Beneficial effects of cold atmospheric plasma on inflammatory phase of diabetic foot ulcers; a randomized clinical trial. J Diabetes Metabolic Disorders. 2020;19(2):895–905.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1038/s41598-017-15725-7",
"author": "YS Lee",
"doi-asserted-by": "publisher",
"first-page": "15564",
"issue": "1",
"journal-title": "SCI REP-UK",
"key": "9886_CR39",
"unstructured": "Lee YS, Lee M, Kim H, Won H, Kim C. Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression. Sci Rep-UK. 2017;7(1):15564.",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1111/odi.13697",
"author": "C Seebauer",
"doi-asserted-by": "publisher",
"first-page": "1728",
"issue": "7",
"journal-title": "ORAL DIS",
"key": "9886_CR40",
"unstructured": "Seebauer C, Freund E, Hasse S, Miller V, Segebarth M, Lucas C, Kindler S, Dieke T, Metelmann HR, Daeschlein G, et al. Effects of cold physical plasma on oral lichen planus: an in vitro study (effects of CAP on OLP). Oral Dis. 2021;27(7):1728–37.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.jid.2021.04.019",
"author": "S Zhai",
"doi-asserted-by": "publisher",
"first-page": "2710",
"issue": "11",
"journal-title": "J INVEST DERMATOL",
"key": "9886_CR41",
"unstructured": "Zhai S, Xu M, Li Q, Guo K, Chen H, Kong MG, Xia Y. Successful treatment of vitiligo with cold atmospheric plasma–activated hydrogel. J Invest Dermatol. 2021;141(11):2710–9.",
"volume": "141",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-802084/v1",
"doi-asserted-by": "crossref",
"key": "9886_CR42",
"unstructured": "Zhang W, Chua B, Selva K, Kedzierski L, Ashhurst T, Haycroft E, Shoffner S, Hensen L, Boyd D, James F et al. Immune responses in COVID-19 respiratory tract and blood reveal mechanisms of disease severity. Res Sq. 2021."
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "W Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"issue": "18",
"journal-title": "NEW ENGL J MED",
"key": "9886_CR43",
"unstructured": "Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, Liu L, Shan H, Lei C, Hui DSC, et al. Clinical characteristics of Coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708–20.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "9886_CR44",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1378/chest.129.1_suppl.138S",
"author": "SS Braman",
"doi-asserted-by": "publisher",
"first-page": "S138",
"issue": "1",
"journal-title": "Chest",
"key": "9886_CR45",
"unstructured": "Braman SS. Postinfectious cough. Chest. 2006;129(1):S138–46.",
"volume": "129",
"year": "2006"
},
{
"DOI": "10.1016/S2213-2600(21)00125-9",
"author": "W Song",
"doi-asserted-by": "publisher",
"first-page": "533",
"issue": "5",
"journal-title": "Lancet Respiratory Med",
"key": "9886_CR46",
"unstructured": "Song W, Hui CKM, Hull JH, Birring SS, McGarvey L, Mazzone SB, Chung KF. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respiratory Med. 2021;9(5):533–44.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.imlet.2019.12.013",
"author": "L Tamasauskiene",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "IMMUNOL LETT",
"key": "9886_CR47",
"unstructured": "Tamasauskiene L, Sitkauskiene B. Immune system in the pathogenesis of chronic cough. Immunol Lett. 2020;218:40–3.",
"volume": "218",
"year": "2020"
},
{
"DOI": "10.1038/s41598-019-49938-9",
"doi-asserted-by": "crossref",
"key": "9886_CR48",
"unstructured": "Lee M, Lee YS, Kim HJ, Han CH, Kang SU, Kim C. Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice. Sci Rep-UK. 2019;9(1)."
},
{
"DOI": "10.1038/srep27376",
"doi-asserted-by": "crossref",
"key": "9886_CR49",
"unstructured": "Choi J, Song Y, Lee H, Hong J, Kim G. Inhibition of inflammatory reactions in 2,4-dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Sci Rep-UK. 2016;6(1)."
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09886-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Nebulized inhalation of plasma-activated water in the treatment of progressive moderate COVID-19 patients with antiviral treatment failure: a randomized controlled pilot trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}