Efficacy of Nirmatrelvir-Ritonavir versus Azvudine for COVID-19 Treatment in Tibet: A Retrospective Study
et al., Infection and Drug Resistance, doi:10.2147/IDR.S423725, Sep 2023
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
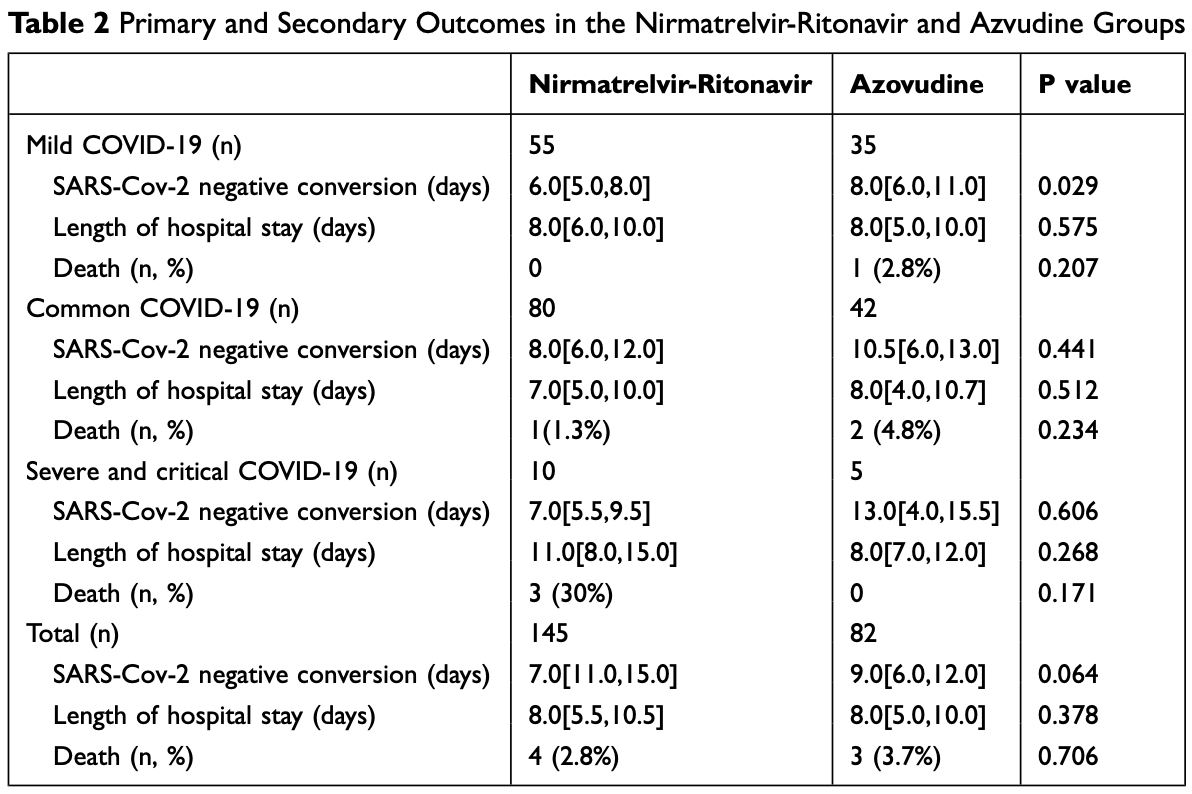
Retrospective propensity-matched analysis of 227 COVID-19 patients in Tibet, China comparing azvudine to paxlovid. Overall, azvudine had comparable viral clearance time and clinical outcomes to paxlovid. However, for mild COVID-19 cases, paxlovid had faster viral clearance than azvudine (6 vs 8 days, p=0.029). No significant differences were seen in length of hospitalization or mortality rate between the groups.
Zhao et al., 30 Sep 2023, retrospective, China, peer-reviewed, 9 authors, study period 5 June, 2023 - 2 July, 2023.
Contact: softsnake@bjmu.edu.cn.
Efficacy of Nirmatrelvir-Ritonavir versus Azvudine for COVID-19 Treatment in Tibet: A Retrospective Study
Infection and Drug Resistance, doi:10.2147/idr.s423725
Background: Nirmatrelvir-ritonavir, also known as paxlovid, is a widely used antiviral drug against coronavirus disease 2019 (COVID-19). Azvudine, a drug previously used to treat human immunodeficiency virus-1, has also been used to treat COVID-19 in China. However, only a few clinical studies have evaluated the effects of azvudine. Additionally, studies comparing nirmatrelvirritonavir with azvudine have been limited in number. Methods: We carried out a retrospective case-control analysis at the Third People's Hospital of the Tibet Autonomous Region. Eighty-two eligible patients with COVID-19 who received azvudine treatment were included. A total of 145 control patients who received nirmatrelvir-ritonavir treatment were selected by propensity score matching for age, sex, the severity of disease, and initial cycle threshold values. A comparison of the nucleic acid test negative conversion time, the length of hospitalization, and mortality rate was conducted. Results: Overall, the mean nucleic acid test negative conversion time was comparable between the nirmatrelvir-ritonavir and azvudine groups (7.0 [11.0, 15.0] vs 9.0 [6.0, 12.0] days, P=0.064). However, for patients with mild COVID-19, the nucleic acid test negative conversion time was significantly shorter in the nirmatrelvir-ritonavir group than in the azvudine group (6.0 [5.0, 8.0] vs 8.0 [6.0, 11.0] days, P=0.029). The nirmatrelvir-ritonavir group and the azvudine group did not differ significantly in length of hospitalization (8.0 [5.5,10.5] vs 8.0 [5.0,10.0] days, P=0.378). Regarding the mortality rate, there were 4 (2.8%) deaths in the nirmatrelvir-ritonavir group and 3 (3.7%) in the azvudine group (P=0.706).
Conclusion: Azvudine is generally as effective as nirmatrelvir-ritonavir, but for patients with mild COVID-19, nirmatrelvir-ritonavir could suppress the virus more rapidly. For those who cannot be treated with nirmatrelvir-ritonavir, azvudine might be an effective therapy for COVID-19.
Patient Consent Statement The research on patients was approved by the Third People's Hospital of the Tibet Autonomous Region Clinical Research Ethics Committee, approval number ME-TBHP-23-03. All patients in the retrospective cohort study were anonymous, and the individual informed consent was not required. The Third People's Hospital of the Tibet Autonomous Region specifically waived the requirement for informed consent.
Author Contributions All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all the following areas; took part in drafting, revised or critically reviewed the article; approved final version of the manuscript; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure The authors declare no conflicts of interest in this work.
References
Arias-Reyes, Carvajal-Rodriguez, Poma-Machicao, Decreased incidence, virus transmission capacity, and severity of COVID-19 at altitude on the American continent, PLoS One, doi:10.1371/journal.pone.0237294
Beasley, Price of COVID treatments from Pfizer, Merck, GSK align with patient benefits-report, Reuters Healthc Pharmaceuticals
Da Silva, Cabral, Souza, Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front Med, doi:10.3389/fmed.2023.1143485
Fernandes, Inchakalody, Merhi, Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines, Ann Med, doi:10.1080/07853890.2022.2031274
Gao, Luo, Ren, Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, J Infect, doi:10.1016/j.jinf.2023.03.023
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Lee, Suzuki, COVID-19: variants, immunity, and therapeutics for non-hospitalized patients, Biomedicines, doi:10.3390/biomedicines11072055
Lei, Chen, Wu, Duan, Men, Small molecules in the treatment of COVID-19, Signal Transduct Target Ther, doi:10.1038/s41392-022-01249-8
Liu, Wang, Zhou, Zhao, Zhang et al., Potential Role of ACE2 in Coronavirus Disease 2019 (COVID-19) Prevention and Management, J Transl Int Med, doi:10.2478/jtim-2020-0003
Marzolini, Kuritzkes, Marra, Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications, Clin Pharmacol Ther, doi:10.1002/cpt.2646
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Ren, Luo, Yu, A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study, Adv Sci, doi:10.1002/advs.202001435
Segovia-Juarez, Castagnetto, Gonzales, High altitude reduces infection rate of COVID-19 but not case-fatality rate, Respir Physiol Neurobiol, doi:10.1016/j.resp.2020.103494
Shang, Pan, Yang, Expert Consensus on the Diagnosis and Treatment of Critically Ill Patients with COVID-19 at High Altitudes in China, Chin J Crit Care Intensive Care Med
Steele, Couture, Reed, Estimated Number of COVID-19 Infections, Hospitalizations, and Deaths Prevented Among Vaccinated Persons in the US, December 2020 to September 2021, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.20385
Stephens, Chernyavskiy, Bruns, Impact of altitude on COVID-19 infection and death in the United States: a modeling and observational study, PLoS One, doi:10.1371/journal.pone.0245055
Sun, Dian, epidemiology of antibiotic resistance and the mechanisms of resistance development and diffusion in both hospitals and the community, doi:10.1016/j.eclinm.2023.101981
Wang, Zhao, Chen, Liu, Feng, The effect of nirmatrelvir-ritonavir on viral clearance and length of hospital stay in patients infected with SARS-CoV-2 omicron variants, Influenza Other Respir Viruses, doi:10.1111/irv.13095
Yang, Wang, Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China, Eur J Med Chem, doi:10.1016/j.ejmech.2023.115503
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct Target Ther, doi:10.1038/s41392-020-00351-z
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation, doi:10.1016/j.xinn.2022.100321
Zhang, Li, Wang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00835-6
Zheng, Yu, Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study, BMJ, doi:10.1136/bmj.m1443
DOI record:
{
"DOI": "10.2147/idr.s423725",
"ISSN": [
"1178-6973"
],
"URL": "http://dx.doi.org/10.2147/IDR.S423725",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3551-1671",
"affiliation": [],
"authenticated-orcid": true,
"family": "Zhao",
"given": "Xiang",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-9763-7424",
"affiliation": [],
"authenticated-orcid": true,
"family": "Cheng",
"given": "Yuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Meng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qianda",
"given": "Bianba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhouma",
"given": "Baima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yangzhen",
"given": "Bianba",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Yao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Shuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Huiying",
"sequence": "additional"
}
],
"container-title": "Infection and Drug Resistance",
"container-title-short": "IDR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
9,
11
]
],
"date-time": "2023-09-11T04:45:15Z",
"timestamp": 1694407515000
},
"deposited": {
"date-parts": [
[
2023,
9,
11
]
],
"date-time": "2023-09-11T04:45:20Z",
"timestamp": 1694407520000
},
"indexed": {
"date-parts": [
[
2023,
9,
12
]
],
"date-time": "2023-09-12T22:42:03Z",
"timestamp": 1694558523324
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T00:00:00Z",
"timestamp": 1693526400000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=92683",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=92683",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "6053-6060",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2023,
9
]
]
},
"published-online": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1001/jamanetworkopen.2022.20385",
"author": "Steele",
"doi-asserted-by": "publisher",
"first-page": "e2220385",
"journal-title": "JAMA Netw Open",
"key": "ref1",
"volume": "5",
"year": "2022"
},
{
"key": "ref2",
"unstructured": "Weekly epidemiological update on COVID-19 - 27 April 2023. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---27-april-2023. Accessed September 6, 2023."
},
{
"DOI": "10.1080/07853890.2022.2031274",
"author": "Fernandes",
"doi-asserted-by": "publisher",
"first-page": "524",
"journal-title": "Ann Med",
"key": "ref3",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"author": "Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "e342",
"journal-title": "Clin Infect Dis",
"key": "ref4",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "ref5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1111/irv.13095",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "e13095",
"journal-title": "Influenza Other Respir Viruses",
"key": "ref6",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1002/cpt.2646",
"author": "Marzolini",
"doi-asserted-by": "publisher",
"first-page": "1191",
"journal-title": "Clin Pharmacol Ther",
"key": "ref7",
"volume": "112",
"year": "2022"
},
{
"author": "Beasley",
"first-page": "56",
"journal-title": "Reuters Healthc Pharmaceuticals",
"key": "ref8",
"year": "2022"
},
{
"DOI": "10.3390/biomedicines11072055",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "2055",
"journal-title": "Biomedicines",
"key": "ref9",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "100321",
"journal-title": "Innovation",
"key": "ref10",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "236",
"journal-title": "Signal Transduct Target Ther",
"key": "ref11",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal Transduct Target Ther",
"key": "ref12",
"volume": "6",
"year": "2021"
},
{
"key": "ref13",
"unstructured": "National Health Commission of the People’s Republic of China. Diagnosis and treatment plan for COVID-19 (trial version 9. Int J Epidemiol Infect Dis. 2022;49:73–80."
},
{
"author": "Shang",
"first-page": "1",
"journal-title": "Chin J Crit Care Intensive Care Med",
"key": "ref14",
"year": "2020"
},
{
"DOI": "10.1038/s41392-022-01249-8",
"author": "Lei",
"doi-asserted-by": "publisher",
"first-page": "387",
"journal-title": "Signal Transduct Target Ther",
"key": "ref15",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2023.115503",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "115503",
"journal-title": "Eur J Med Chem",
"key": "ref16",
"volume": "257",
"year": "2023"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"journal-title": "Adv Sci",
"key": "ref17",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"author": "da Silva",
"doi-asserted-by": "publisher",
"first-page": "1143485",
"journal-title": "Front Med",
"key": "ref18",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "e158",
"journal-title": "J Infect",
"key": "ref19",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1016/j.resp.2020.103494",
"author": "Segovia-Juarez",
"doi-asserted-by": "publisher",
"first-page": "103494",
"journal-title": "Respir Physiol Neurobiol",
"key": "ref20",
"volume": "281",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0237294",
"author": "Arias-Reyes",
"doi-asserted-by": "publisher",
"first-page": "e0237294",
"journal-title": "PLoS One",
"key": "ref21",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.2478/jtim-2020-0003",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "J Transl Int Med",
"key": "ref22",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0245055",
"author": "Stephens",
"doi-asserted-by": "publisher",
"first-page": "e0245055",
"journal-title": "PLoS One",
"key": "ref23",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m1443",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "m1443",
"journal-title": "BMJ",
"key": "ref24",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "101981",
"journal-title": "EClinicalMedicine",
"key": "ref25",
"volume": "59",
"year": "2023"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/efficacy-of-nirmatrelvir-ritonavir-versus-azvudine-for-covid-19-treatm-peer-reviewed-fulltext-article-IDR"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Pharmacology"
],
"subtitle": [],
"title": "Efficacy of Nirmatrelvir-Ritonavir versus Azvudine for COVID-19 Treatment in Tibet: A Retrospective Study",
"type": "journal-article",
"volume": "Volume 16"
}
