Inflammatory predictors (eosinophil, C-RP and IL-6) and effectiveness of oral Azvudine tablets treatment in COVID-19 hospitalized patients: A retrospective, self-controlled study
et al., Heliyon, doi:10.1016/j.heliyon.2023.e21941, Nov 2023
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
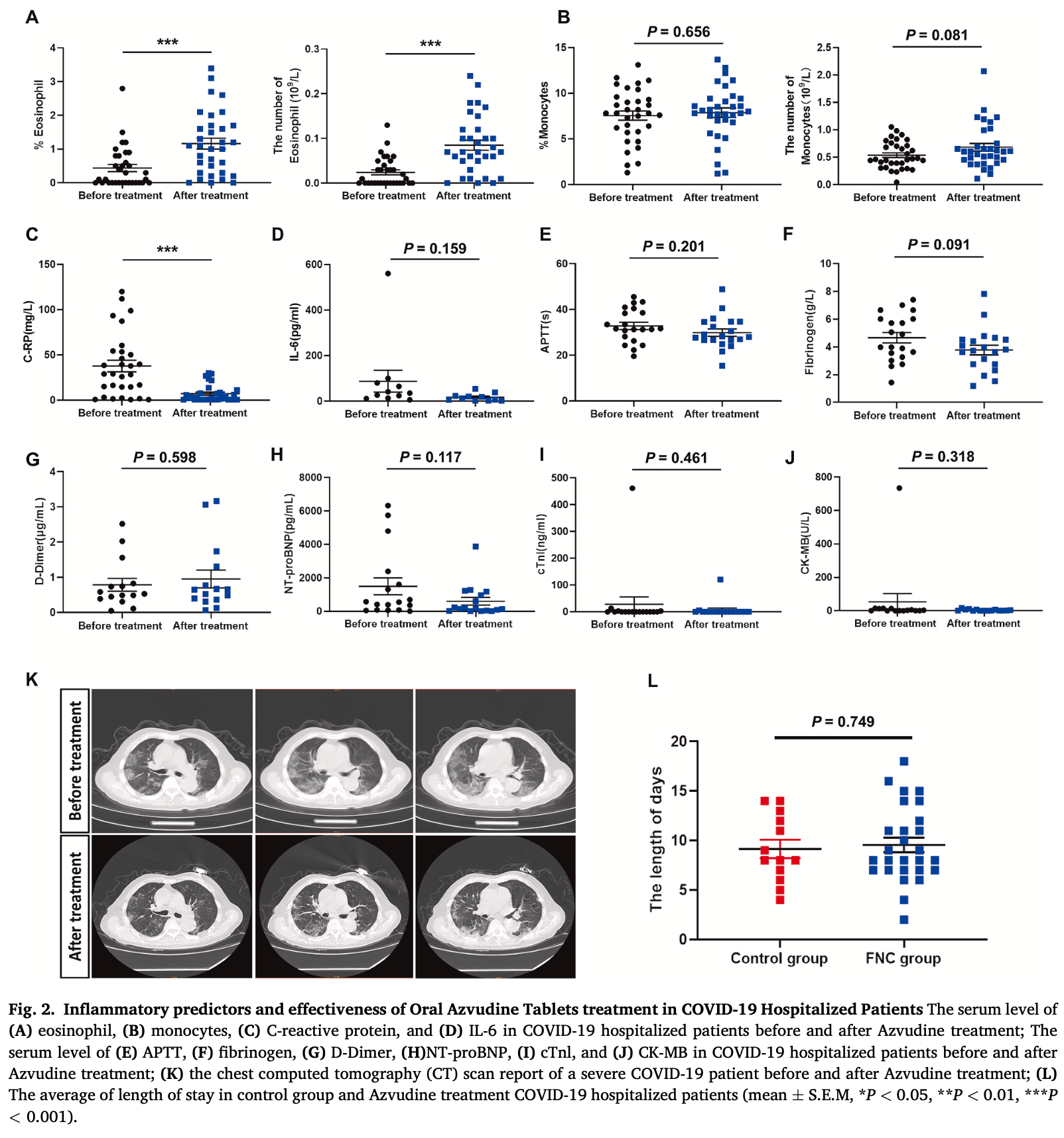
Retrospective 60 hospitalized COVID-19 patients in China, 32 treated with azvudine for 7-14 days. The azvudine group had impoved eosinophil counts, CRP, IL-6, fibrinogen, NT-proBNP, and improved lung CT findings, suggesting reduced inflammation and improved coagulation function. However, there was no difference in length of stay compared to controls. Authors do not provide baseline information for the control group, preventing accurate interpretation of the results.
Zhao et al., 7 Nov 2023, retrospective, China, peer-reviewed, 6 authors.
Contact: yanlizhao2015@126.com, qhschangrong@126.com.
Inflammatory predictors (eosinophil, C-RP and IL-6) and effectiveness of oral Azvudine tablets treatment in COVID-19 hospitalized patients: A retrospective, self-controlled study
Heliyon, doi:10.1016/j.heliyon.2023.e21941
Background: Although vaccinations and antiviral drugs are widely used in the clinical treatment worldwide, there is little investigation on the clinical outcomes and effectiveness of oral Azvudine tablets (FNC) treatment in COVID-19 hospitalized patients. The previous data showed Azvudine treatment was closely related to reduced virus shedding time, but the potential role of Azvudine on inflammatory response is scarce. Thus, this study is to investigate inflammatory predictors and effectiveness of oral Azvudine tablets treatment in COVID-19 hospitalized patients. Methods: A total of 600 out of hospitalized patients were retrospectively collected over a 2-month period, of whom 60 out of hospitalized patients infected SARS-CoV-2. 32 of hospitalized patients who received Azvudine tablets were collected and the rest did not. Oral Azvudine tablets treatment: 5 mg/day for 7-14 days. We analyzed the routine blood tests, blood coagulation test, NT-proBNP, Troponin (cTNl), Creatine kinase MB (CK-MB) after oral Azvudine tablets treatment compared with that in before oral Azvudine tablets treatment. Also, we compared the CT chest and length of Stay after Azvudine treatment. Results: We found that the number and percentage of eosinophil increased significantly, but the levels of C-reactive protein (C-RP) and IL-6 reduced remarkably after Azvudine treatment. In blood coagulation tests, the results showed that activated partial thromboplastin time (APTT) (mean ± SEM: 2.950 ± 2.268s) and fibrinogen (mean ± SEM: 0.8910 ± 0.5134g/L) downregulated slightly, while there was similar in the level of D-Dimer (mean ± SEM: 0.1660 ± 0.3108 μg/mL) before and after Azvudine treatment. The expression of NT-proBNP reduced in Azvudine treatment (mean ± SEM: 897.1 ± 557.1pg/mL). Chest computed tomography (CT) scan reports also demonstrated that Azvudine treatment improved lung symptoms in COVID-19 hospitalized patients. Moreover, there is no difference in the average of length of stay in Azvudine treatment (the average of LOS days: 9.0) and no treatment (the average of LOS days: 9.0) Conclusion: Oral Azvudine tablets treatment was associated with decreased inflammatory response and improved blood coagulation function, which should be substantial clinical benefits in COVID-19 hospitalized patients.
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Andrews, Tessier, Stowe, Gower, Kirsebom et al., Duration of protection against mild and severe disease by covid-19 vaccines, N. Engl. J. Med
Caro-Codon, Rey, Buno, Iniesta, Rosillo et al., Characterization of NT-proBNP in a large cohort of COVID-19 patients, Eur. J. Heart Fail
Chen, Xu, Hong, Yang, Peng et al., Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study, medRxiv
Da Silva, Abreu Cabral, De Souza, Arruda, Cabral et al., Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front. Med
Dale, Takhar, Carragher, Katsoulis, Torabi et al., The impact of the COVID-19 pandemic on cardiovascular disease prevention and management, Nat. Med
Gao, Jiang, Wen, Cheng, Sun et al., Prognostic value of NT-proBNP in patients with severe COVID-19, Respir. Res
Guo, Fan, Chen, Wu, Zhang et al., Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19), JAMA Cardiol
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19, N. Engl. J. Med
Herold, Jurinovic, Arnreich, Lipworth, Hellmuth et al., Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19, J. Allergy Clin. Immunol
Kudlinski, Zgola, Stolinska, Murkos, Kania et al., Systemic inflammatory predictors of in-hospital mortality in COVID-19 patients: a retrospective study, Diagnostics
Lau, Cheng, Leung, Lee, Hachim et al., Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population, Nat. Med
Leonard-Lorant, Delabranche, Severac, Helms, Pauzet et al., Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels, Radiology
Li, Ding, Xia, Chen, Chen et al., Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case-control study, EClinicalMedicine
Liu, Li, Xu, Wu, Luo et al., Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19, J. Clin. Virol
Mangalmurti, Hunter, Cytokine storms: understanding COVID-19, Immunity
Michot, Albiges, Chaput, Saada, Pommeret et al., Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report, Ann. Oncol
Milenkovic, Hadzibegovic, Kovac, Jovanovic, Stanisavljevic et al., PCT, and IL-6 levels at admission to ICU can predict in-hospital mortality in patients with COVID-19 pneumonia, Oxid. Med. Cell. Longev
Mu, Yi, Wang, Wang, Zhang et al., Expression of eosinophil in peripheral blood of patients with COVID-19 and its clinical significance, J. Clin. Lab. Anal
Murakami, Hayden, Hills, Al-Samkari, Casey et al., Therapeutic advances in COVID-19, Nat. Rev. Nephrol
Ponikowski, Voors, Anker, Bueno, Cleland et al., ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC, Eur. J. Heart Fail
Ren, Luo, Yu, Song, Liang et al., A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv. Sci
Rostami, Mansouritorghabeh, D-dimer level in COVID-19 infection: a systematic review, Expert Rev. Hematol
Shen, Xiao, Sun, Li, Wu et al., Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study, medRxiv
Tadic, Cuspidi, Mancia, Dell'oro, Grassi, COVID-19, hypertension and cardiovascular diseases: should we change the therapy?, Pharmacol. Res
Varga, Flammer, Steiger, Haberecker, Andermatt et al., Endothelial cell infection and endotheliitis in COVID-19, Lancet
Wang, Yang, Post-acute sequelae of SARS-CoV-2 infection: a neglected public Health issue, Front. Public Health
Yang, Wang, Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China, Eur. J. Med. Chem
Yao, Cao, Wang, Shi, Liu et al., D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study, J Intensive Care
Ye, China approves first homegrown COVID antiviral, Nature
Yip, Lui, Lai, Wong, Tse et al., Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19), Clin. Infect. Dis
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct, Targeted Ther
Zeng, Wang, Li, Zhang, Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19, J. Med. Virol
Zhang, Jiao, Li, Yu, Pei et al., Elevated INR in a COVID-19 patient after concomitant administration of azvudine and anticoagulants, Front. Pharmacol
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct, Targeted Ther
Zhao, None, Heliyon
Zhou, Chi, Lv, Wang, Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19), Diabetes Metab Res Rev
Zou, Guo, Zhang, Zhang, Liu et al., Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China, Biosci Trends
DOI record:
{
"DOI": "10.1016/j.heliyon.2023.e21941",
"ISSN": [
"2405-8440"
],
"URL": "http://dx.doi.org/10.1016/j.heliyon.2023.e21941",
"alternative-id": [
"S2405844023091491"
],
"article-number": "e21941",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Inflammatory predictors (eosinophil, C-RP and IL-6) and effectiveness of oral Azvudine tablets treatment in COVID-19 hospitalized patients: A retrospective, self-controlled study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Heliyon"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.heliyon.2023.e21941"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-9445-7666",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhao",
"given": "Yanli",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gao",
"given": "Gan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Wenhui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Zuqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Rong",
"sequence": "additional"
}
],
"container-title": "Heliyon",
"container-title-short": "Heliyon",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
11,
7
]
],
"date-time": "2023-11-07T08:06:50Z",
"timestamp": 1699344410000
},
"deposited": {
"date-parts": [
[
2023,
11,
25
]
],
"date-time": "2023-11-25T05:53:34Z",
"timestamp": 1700891614000
},
"indexed": {
"date-parts": [
[
2023,
11,
26
]
],
"date-time": "2023-11-26T00:11:15Z",
"timestamp": 1700957475130
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844023091491?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2405844023091491?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e21941",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1002/dmrr.3377",
"article-title": "Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19)",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "e3377",
"issue": "2",
"journal-title": "Diabetes Metab Res Rev",
"key": "10.1016/j.heliyon.2023.e21941_bib1",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-02158-7",
"article-title": "The impact of the COVID-19 pandemic on cardiovascular disease prevention and management",
"author": "Dale",
"doi-asserted-by": "crossref",
"first-page": "219",
"issue": "1",
"journal-title": "Nat. Med.",
"key": "10.1016/j.heliyon.2023.e21941_bib2",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1016/j.phrs.2020.104906",
"article-title": "COVID-19, hypertension and cardiovascular diseases: should we change the therapy?",
"author": "Tadic",
"doi-asserted-by": "crossref",
"journal-title": "Pharmacol. Res.",
"key": "10.1016/j.heliyon.2023.e21941_bib3",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciac687",
"article-title": "Impact of the use of oral antiviral agents on the risk of hospitalization in community coronavirus disease 2019 patients (COVID-19)",
"author": "Yip",
"doi-asserted-by": "crossref",
"first-page": "e26",
"issue": "3",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.heliyon.2023.e21941_bib4",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2115481",
"article-title": "Duration of protection against mild and severe disease by covid-19 vaccines",
"author": "Andrews",
"doi-asserted-by": "crossref",
"first-page": "340",
"issue": "4",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.heliyon.2023.e21941_bib5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41591-023-02219-5",
"article-title": "Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population",
"author": "Lau",
"doi-asserted-by": "crossref",
"first-page": "348",
"issue": "2",
"journal-title": "Nat. Med.",
"key": "10.1016/j.heliyon.2023.e21941_bib6",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.heliyon.2023.e21941_bib7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/d41586-022-02050-x",
"article-title": "China approves first homegrown COVID antiviral",
"author": "Ye",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "10.1016/j.heliyon.2023.e21941_bib8",
"year": "2022"
},
{
"DOI": "10.1038/s41581-022-00642-4",
"article-title": "Therapeutic advances in COVID-19",
"author": "Murakami",
"doi-asserted-by": "crossref",
"first-page": "38",
"issue": "1",
"journal-title": "Nat. Rev. Nephrol.",
"key": "10.1016/j.heliyon.2023.e21941_bib9",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1016/j.ejmech.2023.115503",
"article-title": "Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China",
"author": "Yang",
"doi-asserted-by": "crossref",
"journal-title": "Eur. J. Med. Chem.",
"key": "10.1016/j.heliyon.2023.e21941_bib10",
"volume": "257",
"year": "2023"
},
{
"article-title": "Post-acute sequelae of SARS-CoV-2 infection: a neglected public Health issue",
"author": "Wang",
"journal-title": "Front. Public Health",
"key": "10.1016/j.heliyon.2023.e21941_bib11",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.annonc.2020.03.300",
"article-title": "Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report",
"author": "Michot",
"doi-asserted-by": "crossref",
"first-page": "961",
"issue": "7",
"journal-title": "Ann. Oncol.",
"key": "10.1016/j.heliyon.2023.e21941_bib12",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100375",
"article-title": "Eosinopenia and elevated C-reactive protein facilitate triage of COVID-19 patients in fever clinic: a retrospective case-control study",
"author": "Li",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.heliyon.2023.e21941_bib13",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1002/jcla.23620",
"article-title": "Expression of eosinophil in peripheral blood of patients with COVID-19 and its clinical significance",
"author": "Mu",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "J. Clin. Lab. Anal.",
"key": "10.1016/j.heliyon.2023.e21941_bib14",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"article-title": "Azvudine (FNC): a promising clinical candidate for COVID-19 treatment",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "236",
"issue": "1",
"journal-title": "Signal Transduct. Targeted Ther.",
"key": "10.1016/j.heliyon.2023.e21941_bib15",
"volume": "5",
"year": "2020"
},
{
"article-title": "Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study",
"author": "Chen",
"first-page": "2023",
"journal-title": "medRxiv",
"key": "10.1016/j.heliyon.2023.e21941_bib16",
"year": "2023"
},
{
"article-title": "Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Shen",
"first-page": "2023",
"journal-title": "medRxiv",
"key": "10.1016/j.heliyon.2023.e21941_bib17",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct. Targeted Ther.",
"key": "10.1016/j.heliyon.2023.e21941_bib18",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3390/diagnostics12040859",
"article-title": "Systemic inflammatory predictors of in-hospital mortality in COVID-19 patients: a retrospective study",
"author": "Kudlinski",
"doi-asserted-by": "crossref",
"first-page": "859",
"issue": "4",
"journal-title": "Diagnostics",
"key": "10.1016/j.heliyon.2023.e21941_bib19",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.immuni.2020.06.017",
"article-title": "Cytokine storms: understanding COVID-19",
"author": "Mangalmurti",
"doi-asserted-by": "crossref",
"first-page": "19",
"issue": "1",
"journal-title": "Immunity",
"key": "10.1016/j.heliyon.2023.e21941_bib20",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study",
"author": "Ren",
"doi-asserted-by": "crossref",
"issue": "19",
"journal-title": "Adv. Sci.",
"key": "10.1016/j.heliyon.2023.e21941_bib21",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"article-title": "Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19",
"author": "da Silva",
"doi-asserted-by": "crossref",
"journal-title": "Front. Med.",
"key": "10.1016/j.heliyon.2023.e21941_bib22",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28836",
"article-title": "Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19",
"author": "Zeng",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.heliyon.2023.e21941_bib23",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1155/2022/8997709",
"article-title": "PCT, and IL-6 levels at admission to ICU can predict in-hospital mortality in patients with COVID-19 pneumonia",
"author": "Milenkovic",
"doi-asserted-by": "crossref",
"journal-title": "Oxid. Med. Cell. Longev.",
"key": "10.1016/j.heliyon.2023.e21941_bib24",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.1016/j.jaci.2020.05.008",
"article-title": "Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19",
"author": "Herold",
"doi-asserted-by": "crossref",
"first-page": "128",
"issue": "1",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "10.1016/j.heliyon.2023.e21941_bib25",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2020.104370",
"article-title": "Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"journal-title": "J. Clin. Virol.",
"key": "10.1016/j.heliyon.2023.e21941_bib26",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"article-title": "Endothelial cell infection and endotheliitis in COVID-19",
"author": "Varga",
"doi-asserted-by": "crossref",
"first-page": "1417",
"issue": "10234",
"journal-title": "Lancet",
"key": "10.1016/j.heliyon.2023.e21941_bib27",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.5582/bst.2020.03086",
"article-title": "Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China",
"author": "Zou",
"doi-asserted-by": "crossref",
"first-page": "285",
"issue": "4",
"journal-title": "Biosci Trends",
"key": "10.1016/j.heliyon.2023.e21941_bib28",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1148/radiol.2020201561",
"article-title": "Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels",
"author": "Leonard-Lorant",
"doi-asserted-by": "crossref",
"first-page": "E189",
"issue": "3",
"journal-title": "Radiology",
"key": "10.1016/j.heliyon.2023.e21941_bib29",
"volume": "296",
"year": "2020"
},
{
"DOI": "10.1186/s40560-020-00466-z",
"article-title": "D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "J Intensive Care",
"key": "10.1016/j.heliyon.2023.e21941_bib30",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1080/17474086.2020.1831383",
"article-title": "D-dimer level in COVID-19 infection: a systematic review",
"author": "Rostami",
"doi-asserted-by": "crossref",
"first-page": "1265",
"issue": "11",
"journal-title": "Expert Rev. Hematol.",
"key": "10.1016/j.heliyon.2023.e21941_bib31",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1002/ejhf.592",
"author": "Ponikowski",
"doi-asserted-by": "crossref",
"first-page": "891",
"issue": "8",
"journal-title": "Eur. J. Heart Fail.",
"key": "10.1016/j.heliyon.2023.e21941_bib32",
"volume": "18",
"year": "2016"
},
{
"DOI": "10.1002/ejhf.2095",
"article-title": "Characterization of NT-proBNP in a large cohort of COVID-19 patients",
"author": "Caro-Codon",
"doi-asserted-by": "crossref",
"first-page": "456",
"issue": "3",
"journal-title": "Eur. J. Heart Fail.",
"key": "10.1016/j.heliyon.2023.e21941_bib33",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1001/jamacardio.2020.1017",
"article-title": "Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19)",
"author": "Guo",
"doi-asserted-by": "crossref",
"first-page": "811",
"issue": "7",
"journal-title": "JAMA Cardiol",
"key": "10.1016/j.heliyon.2023.e21941_bib34",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1186/s12931-020-01352-w",
"article-title": "Prognostic value of NT-proBNP in patients with severe COVID-19",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "83",
"issue": "1",
"journal-title": "Respir. Res.",
"key": "10.1016/j.heliyon.2023.e21941_bib35",
"volume": "21",
"year": "2020"
},
{
"article-title": "Elevated INR in a COVID-19 patient after concomitant administration of azvudine and anticoagulants",
"author": "Zhang",
"journal-title": "Front. Pharmacol.",
"key": "10.1016/j.heliyon.2023.e21941_bib36",
"volume": "14",
"year": "2023"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2405844023091491"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Inflammatory predictors (eosinophil, C-RP and IL-6) and effectiveness of oral Azvudine tablets treatment in COVID-19 hospitalized patients: A retrospective, self-controlled study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "9"
}
